当前位置:
X-MOL 学术
›
Hypertens. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N6-adenosine methylation (m6A): a promising new molecular target in hypertension and cardiovascular diseases
Hypertension Research ( IF 4.3 ) Pub Date : 2019-10-02 , DOI: 10.1038/s41440-019-0338-z Arumugam Paramasivam 1 , Jayaseelan Vijayashree Priyadharsini 1 , Subramanian Raghunandhakumar 2
Hypertension Research ( IF 4.3 ) Pub Date : 2019-10-02 , DOI: 10.1038/s41440-019-0338-z Arumugam Paramasivam 1 , Jayaseelan Vijayashree Priyadharsini 1 , Subramanian Raghunandhakumar 2
Affiliation
|
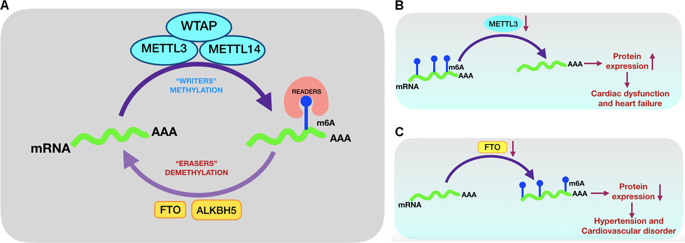
To the Editor: Epitranscriptomics is an emerging field of biology, in which post-transcriptional modifications of RNA, as a novel mechanism discovered recently, are suggested to be a vital process governing the fate and function of RNAs [1]. N6-methyladenosine (m6A) methylation is one of the most abundant and functionally relevant modifications of RNAs, including mRNAs, noncoding RNAs, and microRNAs (miRNAs) [2, 3]. m6A has been shown to play critical roles in many biological processes and a variety of diseases. Being both dynamic and reversible, this modification is a promising new mechanism of posttranscriptional gene regulation. The degree and pattern of m6A modifications on mRNAs can affect their splicing, transport, storage, stability, translation, and decay [3]. The m6A modification is catalyzed by recently discovered enzymes termed writers (m6A methyltransferases, such as METTL3 and METTL14), erasers (m6A demethylases, such as FTO and ALKBH5), and readers (m6A-binding proteins, such as YTHDF1 and IGF2BP1) (Fig. 1a) [1]. Although recent evidence suggests that m6A modifications play a critical role in several diseases, mediating its effect through various mechanisms, the m6A RNA modifications involved in various mechanisms of hypertension and cardiovascular disease remain unknown. Mo et al. showed that m6A plays important roles in blood pressure regulation in a report in Hypertension Research [4]. m6A mRNA methylation regulates cardiac gene expression and cellular growth [5]. Mathiyalagan et al. recently identified m6A as a novel mechanism of human CD34+ stem cell-derived exosomes in cardiac repair [6]. Furthermore, this RNA modification regulates cardiomyocyte Ca2+ dynamics and cardiac function [7]. Dorn et al. recently reported that the normal hypertrophic response in cardiomyocytes is influenced by METTL3mediated m6A methylation of mRNA, which is triggered in response to hypertrophic stimuli. A compensated cardiac hypertrophy results from increased m6A RNA methylation, while the remodeling of cardiomyocytes and their associated dysfunctions are precipitated by diminished m6A methylation (Fig. 1b). Thus, this shuttle of the methylation process has revealed a novel mechanism underlying the stress response for maintaining normal cardiac function [8]. Notably, the m6A modification is reversible. Removal of the m6A modification is catalyzed by FTO (fat mass and obesity-associated protein) RNA demethylase (m6A eraser). Mathiyalagan et al. reported that an increase in m6A was associated with significantly decreased expression of FTO in the ischemic heart [7], and common genetic variants of FTO have been positively associated with blood pressure in patients with hypertension [9] and cardiovascular disease (Fig. 1c) [10]. Recent studies have revealed that aberrant expression of circular RNAs (circRNAs) is related to the occurrence and development of hypertension. Accordingly, circRNAs are proposed as new predictive biomarkers and potential therapeutic targets for different forms of hypertension, including pulmonary hypertension and preeclampsia. m6A, which is the most abundant base modification of circRNAs, promotes efficient initiation of protein translation from circRNAs in human cells. Su et al. generated a transcriptome-wide map of m6A circRNAs in hypoxic pulmonary hypertension (HPH) and identified downregulated or upregulated m6A levels that influenced the circRNA–miRNA–mRNA coexpression network in HPH [3]. Targeting m6A through its writer enzyme METTL3 and eraser enzyme FTO may be used as a potential diagnostic or a novel therapeutic strategy for hypertension and cardiovascular diseases in the future. * Arumugam Paramasivam paramasivam0103@gmail.com
中文翻译:

N6-腺苷甲基化(m6A):高血压和心血管疾病的一个有前途的新分子靶点
致编辑:表观转录组学是一个新兴的生物学领域,其中 RNA 的转录后修饰作为最近发现的一种新机制,被认为是控制 RNA 命运和功能的重要过程 [1]。N6-甲基腺苷 (m6A) 甲基化是 RNA 最丰富和功能相关的修饰之一,包括 mRNA、非编码 RNA 和 microRNA (miRNA) [2, 3]。m6A 已被证明在许多生物过程和多种疾病中发挥关键作用。这种修饰既动态又可逆,是一种很有前途的转录后基因调控新机制。mRNA 上 m6A 修饰的程度和模式会影响其剪接、运输、储存、稳定性、翻译和衰变 [3]。m6A 修饰由最近发现的酶催化,这些酶被称为写入器(m6A 甲基转移酶,例如 METTL3 和 METTL14)、擦除器(m6A 去甲基化酶,例如 FTO 和 ALKBH5)和读取器(m6A 结合蛋白,例如 YTHDF1 和 IGF2BP1)(图. 1a) [1]。尽管最近的证据表明 m6A 修饰在几种疾病中发挥关键作用,通过各种机制调节其作用,但 m6A RNA 修饰参与高血压和心血管疾病的各种机制仍然未知。莫等人。在高血压研究 [4] 的一份报告中表明 m6A 在血压调节中起重要作用。m6A mRNA 甲基化调节心脏基因表达和细胞生长 [5]。马蒂亚拉根等人。最近发现 m6A 是人类 CD34+ 干细胞衍生的外泌体在心脏修复中的一种新机制 [6]。此外,这种 RNA 修饰调节心肌细胞 Ca2+ 动力学和心脏功能 [7]。多恩等人。最近报道,心肌细胞中的正常肥大反应受 METTL3 介导的 mRNA m6A 甲基化的影响,这是响应肥大刺激而触发的。m6A RNA甲基化增加导致代偿性心脏肥大,而m6A甲基化减少则促进心肌细胞的重塑及其相关功能障碍(图1b)。因此,甲基化过程的这种穿梭揭示了维持正常心脏功能的应激反应的新机制[8]。值得注意的是,m6A 修饰是可逆的。m6A 修饰的去除由 FTO(脂肪量和肥胖相关蛋白)RNA 去甲基化酶(m6A 擦除器)催化。马蒂亚拉根等人。据报道,m6A 的增加与缺血性心脏中 FTO 的表达显着降低有关 [7],并且 FTO 的常见遗传变异与高血压 [9] 和心血管疾病患者的血压呈正相关(图 1c) [10]。最近的研究表明,环状RNA(circRNA)的异常表达与高血压的发生发展有关。因此,circRNA 被提议作为不同形式高血压(包括肺动脉高压和先兆子痫)的新预测生物标志物和潜在治疗靶点。m6A 是最丰富的 circRNA 碱基修饰,可促进人类细胞中 circRNA 蛋白质翻译的有效启动。苏等人。生成了缺氧性肺动脉高压 (HPH) 中 m6A circRNA 的全转录组图谱,并确定了影响 HPH 中 circRNA-miRNA-mRNA 共表达网络的下调或上调的 m6A 水平 [3]。通过其写入酶 METTL3 和擦除酶 FTO 靶向 m6A 可用作未来高血压和心血管疾病的潜在诊断或新治疗策略。* Arumugam Paramasivam paramasivam0103@gmail.com
更新日期:2019-10-02
中文翻译:

N6-腺苷甲基化(m6A):高血压和心血管疾病的一个有前途的新分子靶点
致编辑:表观转录组学是一个新兴的生物学领域,其中 RNA 的转录后修饰作为最近发现的一种新机制,被认为是控制 RNA 命运和功能的重要过程 [1]。N6-甲基腺苷 (m6A) 甲基化是 RNA 最丰富和功能相关的修饰之一,包括 mRNA、非编码 RNA 和 microRNA (miRNA) [2, 3]。m6A 已被证明在许多生物过程和多种疾病中发挥关键作用。这种修饰既动态又可逆,是一种很有前途的转录后基因调控新机制。mRNA 上 m6A 修饰的程度和模式会影响其剪接、运输、储存、稳定性、翻译和衰变 [3]。m6A 修饰由最近发现的酶催化,这些酶被称为写入器(m6A 甲基转移酶,例如 METTL3 和 METTL14)、擦除器(m6A 去甲基化酶,例如 FTO 和 ALKBH5)和读取器(m6A 结合蛋白,例如 YTHDF1 和 IGF2BP1)(图. 1a) [1]。尽管最近的证据表明 m6A 修饰在几种疾病中发挥关键作用,通过各种机制调节其作用,但 m6A RNA 修饰参与高血压和心血管疾病的各种机制仍然未知。莫等人。在高血压研究 [4] 的一份报告中表明 m6A 在血压调节中起重要作用。m6A mRNA 甲基化调节心脏基因表达和细胞生长 [5]。马蒂亚拉根等人。最近发现 m6A 是人类 CD34+ 干细胞衍生的外泌体在心脏修复中的一种新机制 [6]。此外,这种 RNA 修饰调节心肌细胞 Ca2+ 动力学和心脏功能 [7]。多恩等人。最近报道,心肌细胞中的正常肥大反应受 METTL3 介导的 mRNA m6A 甲基化的影响,这是响应肥大刺激而触发的。m6A RNA甲基化增加导致代偿性心脏肥大,而m6A甲基化减少则促进心肌细胞的重塑及其相关功能障碍(图1b)。因此,甲基化过程的这种穿梭揭示了维持正常心脏功能的应激反应的新机制[8]。值得注意的是,m6A 修饰是可逆的。m6A 修饰的去除由 FTO(脂肪量和肥胖相关蛋白)RNA 去甲基化酶(m6A 擦除器)催化。马蒂亚拉根等人。据报道,m6A 的增加与缺血性心脏中 FTO 的表达显着降低有关 [7],并且 FTO 的常见遗传变异与高血压 [9] 和心血管疾病患者的血压呈正相关(图 1c) [10]。最近的研究表明,环状RNA(circRNA)的异常表达与高血压的发生发展有关。因此,circRNA 被提议作为不同形式高血压(包括肺动脉高压和先兆子痫)的新预测生物标志物和潜在治疗靶点。m6A 是最丰富的 circRNA 碱基修饰,可促进人类细胞中 circRNA 蛋白质翻译的有效启动。苏等人。生成了缺氧性肺动脉高压 (HPH) 中 m6A circRNA 的全转录组图谱,并确定了影响 HPH 中 circRNA-miRNA-mRNA 共表达网络的下调或上调的 m6A 水平 [3]。通过其写入酶 METTL3 和擦除酶 FTO 靶向 m6A 可用作未来高血压和心血管疾病的潜在诊断或新治疗策略。* Arumugam Paramasivam paramasivam0103@gmail.com











































 京公网安备 11010802027423号
京公网安备 11010802027423号