Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): two double-blind, parallel-group, randomised, controlled phase 3 trials.
The Lancet ( IF 98.4 ) Pub Date : 2019-09-30 , DOI: 10.1016/s0140-6736(19)32215-9 Johann Christian Virchow 1 , Piotr Kuna 2 , Pierluigi Paggiaro 3 , Alberto Papi 4 , Dave Singh 5 , Sandrine Corre 6 , Florence Zuccaro 6 , Andrea Vele 6 , Maxim Kots 6 , George Georges 6 , Stefano Petruzzelli 6 , Giorgio Walter Canonica 7
The Lancet ( IF 98.4 ) Pub Date : 2019-09-30 , DOI: 10.1016/s0140-6736(19)32215-9 Johann Christian Virchow 1 , Piotr Kuna 2 , Pierluigi Paggiaro 3 , Alberto Papi 4 , Dave Singh 5 , Sandrine Corre 6 , Florence Zuccaro 6 , Andrea Vele 6 , Maxim Kots 6 , George Georges 6 , Stefano Petruzzelli 6 , Giorgio Walter Canonica 7
Affiliation
|
BACKGROUND
To date, no studies have assessed the efficacy of single-inhaler triple therapy in asthma. Here we report on two studies that compared the single-inhaler extrafine combination of beclometasone dipropionate (BDP; inhaled corticosteroid), formoterol fumarate (FF; long-acting β2 agonist), and glycopyrronium (G; long-acting muscarinic antagonist) with the combination of BDP with FF.
METHODS
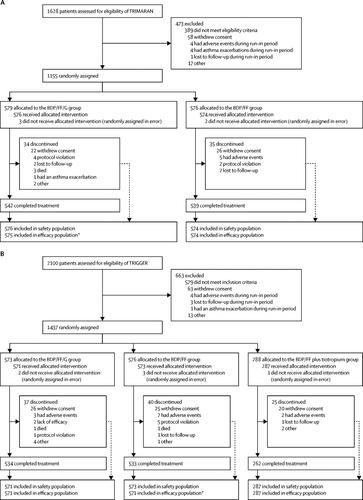
Two parallel-group, double-blind, randomised, active-controlled, phase 3 trials (Triple in Asthma With Uncontrolled Patients on Medium Strength of ICS + LABA [TRIMARAN] and Triple in Asthma High Strength Versus ICS/LABA HS and Tiotropium [TRIGGER]) recruited patients from 171 sites across 16 countries (TRIMARAN), and from 221 sites across 17 countries (TRIGGER). The sites were a mixture of secondary and tertiary care centres and specialised investigation units. Eligible patients were adults (aged 18-75 years) with uncontrolled asthma, a history of one or more exacerbations in the previous year, and previously treated with inhaled corticosteroid (TRIMARAN: medium dose; TRIGGER: high dose) plus a long-acting β2 agonist. Enrolled patients were initially treated with BDP/FF (TRIMARAN: 100 μg BDP and 6 μg FF; TRIGGER: 200 μg BDP and 6 μg FF) for 2 weeks, then randomly assigned to treatment using an interactive response technology system with a balanced block randomisation scheme stratified by country. Patients, investigators, site staff, and sponsor staff were masked to BDP/FF/G and BDP/FF assignment. In TRIMARAN, patients were randomly assigned (1:1) to 52 weeks of BDP/FF/G (100 μg BDP, 6 μg FF, and 10 μg G) or BDP/FF (100 μg BDP and 6 μg FF), two inhalations twice daily. In TRIGGER, patients were randomly assigned (2:2:1) to 52 weeks of BDP/FF/G (200 μg BDP, 6 μg FF, and 10 μg G) or BDP/FF (200 BDP and 6 μg FF), both two inhalations twice daily, or open-label BDP/FF (200 μg BDP and 6 μg FF) two inhalations twice daily plus tiotropium 2·5 μg two inhalations once daily. Coprimary endpoints for both trials (BDP/FF/G vs BDP/FF) were pre-dose forced expiratory volume in 1 s (FEV1) at week 26 and rate of moderate and severe exacerbations over 52 weeks. Safety was assessed in all patients who received at least one dose of study treatment. These trials were registered with ClinicalTrials.gov, NCT02676076 (TRIMARAN), NCT02676089 (TRIGGER).
FINDINGS
Between Feb 17, 2016, and May 17, 2018, 1155 patients in TRIMARAN were given BDP/FF/G (n=579) or BDP/FF (n=576). Between April 6, 2016, and May 28, 2018, 1437 patients in TRIGGER were given BDP/FF/G (n=573), BDP/FF (n=576), or BDP/FF plus tiotropium (n=288). Compared with the BDP/FF group, week 26 predose FEV1 improved in the BDP/FF/G group by 57 mL (95% CI 15-99; p=0·0080) in TRIMARAN and by 73 mL (26-120; p=0·0025) in TRIGGER, with reductions in the rate of moderate and severe exacerbations of 15% (rate ratio 0·85, 95% CI 0·73-0·99; p=0·033) in TRIMARAN and 12% (0·88, 0·75-1·03; p=0·11) in TRIGGER. Four patients had treatment-related serious adverse events, one in TRIMARAN in the BDP/FF/G group and three in TRIGGER-one in the BDP/FF/G and two in the BDP/FF group. Three patients in the BDP/FF/G group in TRIMARAN and two patients in TRIGGER-one in the BDP/FF/G group and one in the BDP/FF group-had adverse events leading to death. None of the deaths were considered as related to treatment.
INTERPRETATION
In uncontrolled asthma, addition of a long-acting muscarinic antagonist to inhaled corticosteroid plus long-acting β2-agonist therapy improves lung function and reduces exacerbations.
FUNDING
Chiesi Farmaceutici.
中文翻译:

非控制性哮喘的单吸入器超细三联疗法(TRIMARAN和TRIGGER):两项双盲,平行组,随机,对照3期试验。
背景技术迄今为止,尚无研究评估单吸入器三联疗法在哮喘中的疗效。在这里,我们报告了两项研究,该研究比较了倍氯米松二丙酸酯(BDP;吸入皮质类固醇),富马酸福莫特罗(FF;长效β2激动剂)和格隆溴铵(G;长效毒蕈碱拮抗剂)的单吸入器超细组合FF的BDP。方法两项平行,双盲,随机,主动控制的3期临床试验(三例哮喘患者,中度ICS + LABA [TRIMARAN]不受控制患者,三例哮喘高强度患者,ICS / LABA HS和噻托溴铵[ [TRIGGER])从16个国家/地区的171个站点(TRIMARAN)和17个国家/地区的221个站点(TRIGGER)招募了患者。这些地点是二级和三级护理中心以及专门调查单位的混合物。符合条件的患者是成年人(18-75岁),患有不受控制的哮喘,在过去一年中有一次或多次加重病史,并且以前接受过吸入糖皮质激素(TRIMARAN:中剂量; TRIGGER:高剂量)加长效β2药物治疗激动剂。入组患者最初接受BDP / FF(TRIMARAN:100μgBDP和6μgFF; TRIGGER:200μgBDP和6μgFF)治疗2周,然后使用具有平衡块随机分配的交互式反应技术系统随机分配至治疗方案按国家分层。患者,研究人员,现场工作人员和申办人员被掩盖在BDP / FF / G和BDP / FF的工作中。在TRIMARAN中,患者被随机分配(1:1)到52周的BDP / FF / G(100μgBDP,6μgFF,和10μgG)或BDP / FF(100μgBDP和6μgFF),每天两次吸入两次。在TRIGGER中,患者被随机分配(2:2:1)52周的BDP / FF / G(200μgBDP,6μgFF和10μgG)或BDP / FF(200 BDP和6μgFF),每天两次吸入两次,或开放标签BDP / FF(200μgBDP和6μgFF)每天两次吸入两次,加上噻托溴铵2·5μg每天两次吸入。两项试验的主要共同终点(BDP / FF / G与BDP / FF)为第26周的用药前强迫呼气量1 s(FEV1),以及52周内中度和重度急性发作率。在接受至少一剂研究治疗药物的所有患者中评估安全性。这些试验已在ClinicalTrials.gov,NCT02676076(TRIMARAN),NCT02676089(TRIGGER)中注册。发现在2016年2月17日至2018年5月17日之间,TRIMARAN的1155例患者接受了BDP / FF / G(n = 579)或BDP / FF(n = 576)。在2016年4月6日至2018年5月28日之间,对1437例TRIGGER患者进行了BDP / FF / G(n = 573),BDP / FF(n = 576)或BDP / FF加噻托溴铵(n = 288)的治疗。与BDP / FF组相比,在TRIMARAN中,BDP / FF / G组第26周给药前FEV1改善了57 mL(95%CI 15-99; p = 0·0080),而73 mL(26-120; p TRIGGER中的比率为= 0·0025),而TRIMARAN的中度和重度急性发作的比率降低了15%(比率比率0·85、95%CI 0·73-0·99; p = 0·033)和12% (0·88,0·75-1·03; p = 0·11)在TRIGGER中。4例患者发生了与治疗相关的严重不良事件,BDP / FF / G组为TRIMARAN,1例为TRIGGER,BDP / FF / G为3例,TRIGRAN为3例,BDP / FF组为2例。TRIMARAN中BDP / FF / G组中的三名患者和TRIGGER中的两名患者,BDP / FF / G组中的一名,BDP / FF组中的一名发生了导致死亡的不良事件。没有一例死亡被认为与治疗有关。解释在不受控制的哮喘中,在吸入性糖皮质激素中加入长效毒蕈碱拮抗剂加上长效β2受体激动剂疗法可改善肺功能并减少病情加重。资金基耶西·法玛切蒂奇(Chiesi Farmaceutici)。
更新日期:2019-11-08
中文翻译:

非控制性哮喘的单吸入器超细三联疗法(TRIMARAN和TRIGGER):两项双盲,平行组,随机,对照3期试验。
背景技术迄今为止,尚无研究评估单吸入器三联疗法在哮喘中的疗效。在这里,我们报告了两项研究,该研究比较了倍氯米松二丙酸酯(BDP;吸入皮质类固醇),富马酸福莫特罗(FF;长效β2激动剂)和格隆溴铵(G;长效毒蕈碱拮抗剂)的单吸入器超细组合FF的BDP。方法两项平行,双盲,随机,主动控制的3期临床试验(三例哮喘患者,中度ICS + LABA [TRIMARAN]不受控制患者,三例哮喘高强度患者,ICS / LABA HS和噻托溴铵[ [TRIGGER])从16个国家/地区的171个站点(TRIMARAN)和17个国家/地区的221个站点(TRIGGER)招募了患者。这些地点是二级和三级护理中心以及专门调查单位的混合物。符合条件的患者是成年人(18-75岁),患有不受控制的哮喘,在过去一年中有一次或多次加重病史,并且以前接受过吸入糖皮质激素(TRIMARAN:中剂量; TRIGGER:高剂量)加长效β2药物治疗激动剂。入组患者最初接受BDP / FF(TRIMARAN:100μgBDP和6μgFF; TRIGGER:200μgBDP和6μgFF)治疗2周,然后使用具有平衡块随机分配的交互式反应技术系统随机分配至治疗方案按国家分层。患者,研究人员,现场工作人员和申办人员被掩盖在BDP / FF / G和BDP / FF的工作中。在TRIMARAN中,患者被随机分配(1:1)到52周的BDP / FF / G(100μgBDP,6μgFF,和10μgG)或BDP / FF(100μgBDP和6μgFF),每天两次吸入两次。在TRIGGER中,患者被随机分配(2:2:1)52周的BDP / FF / G(200μgBDP,6μgFF和10μgG)或BDP / FF(200 BDP和6μgFF),每天两次吸入两次,或开放标签BDP / FF(200μgBDP和6μgFF)每天两次吸入两次,加上噻托溴铵2·5μg每天两次吸入。两项试验的主要共同终点(BDP / FF / G与BDP / FF)为第26周的用药前强迫呼气量1 s(FEV1),以及52周内中度和重度急性发作率。在接受至少一剂研究治疗药物的所有患者中评估安全性。这些试验已在ClinicalTrials.gov,NCT02676076(TRIMARAN),NCT02676089(TRIGGER)中注册。发现在2016年2月17日至2018年5月17日之间,TRIMARAN的1155例患者接受了BDP / FF / G(n = 579)或BDP / FF(n = 576)。在2016年4月6日至2018年5月28日之间,对1437例TRIGGER患者进行了BDP / FF / G(n = 573),BDP / FF(n = 576)或BDP / FF加噻托溴铵(n = 288)的治疗。与BDP / FF组相比,在TRIMARAN中,BDP / FF / G组第26周给药前FEV1改善了57 mL(95%CI 15-99; p = 0·0080),而73 mL(26-120; p TRIGGER中的比率为= 0·0025),而TRIMARAN的中度和重度急性发作的比率降低了15%(比率比率0·85、95%CI 0·73-0·99; p = 0·033)和12% (0·88,0·75-1·03; p = 0·11)在TRIGGER中。4例患者发生了与治疗相关的严重不良事件,BDP / FF / G组为TRIMARAN,1例为TRIGGER,BDP / FF / G为3例,TRIGRAN为3例,BDP / FF组为2例。TRIMARAN中BDP / FF / G组中的三名患者和TRIGGER中的两名患者,BDP / FF / G组中的一名,BDP / FF组中的一名发生了导致死亡的不良事件。没有一例死亡被认为与治疗有关。解释在不受控制的哮喘中,在吸入性糖皮质激素中加入长效毒蕈碱拮抗剂加上长效β2受体激动剂疗法可改善肺功能并减少病情加重。资金基耶西·法玛切蒂奇(Chiesi Farmaceutici)。









































 京公网安备 11010802027423号
京公网安备 11010802027423号