当前位置:
X-MOL 学术
›
Cancer Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic Incorporation of Histone H3 Variants into Chromatin Is Essential for Acquisition of Aggressive Traits and Metastatic Colonization.
Cancer Cell ( IF 48.8 ) Pub Date : 2019-09-26 , DOI: 10.1016/j.ccell.2019.08.006 Ana P Gomes 1 , Didem Ilter 1 , Vivien Low 1 , Adam Rosenzweig 1 , Zih-Jie Shen 2 , Tanya Schild 1 , Martin A Rivas 3 , Ekrem E Er 4 , Dylan R McNally 3 , Anders P Mutvei 1 , Julie Han 1 , Yi-Hung Ou 1 , Paola Cavaliere 5 , Edouard Mullarky 3 , Michal Nagiec 1 , Sejeong Shin 1 , Sang-Oh Yoon 1 , Noah Dephoure 5 , Joan Massagué 4 , Ari M Melnick 3 , Lewis C Cantley 3 , Jessica K Tyler 2 , John Blenis 1
Cancer Cell ( IF 48.8 ) Pub Date : 2019-09-26 , DOI: 10.1016/j.ccell.2019.08.006 Ana P Gomes 1 , Didem Ilter 1 , Vivien Low 1 , Adam Rosenzweig 1 , Zih-Jie Shen 2 , Tanya Schild 1 , Martin A Rivas 3 , Ekrem E Er 4 , Dylan R McNally 3 , Anders P Mutvei 1 , Julie Han 1 , Yi-Hung Ou 1 , Paola Cavaliere 5 , Edouard Mullarky 3 , Michal Nagiec 1 , Sejeong Shin 1 , Sang-Oh Yoon 1 , Noah Dephoure 5 , Joan Massagué 4 , Ari M Melnick 3 , Lewis C Cantley 3 , Jessica K Tyler 2 , John Blenis 1
Affiliation
|
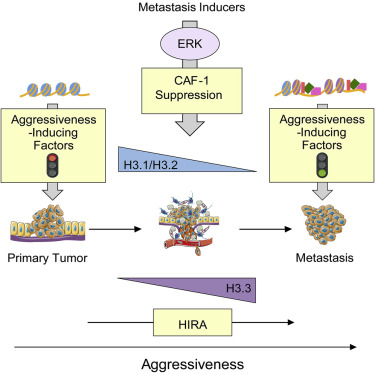
Metastasis is the leading cause of cancer mortality. Chromatin remodeling provides the foundation for the cellular reprogramming necessary to drive metastasis. However, little is known about the nature of this remodeling and its regulation. Here, we show that metastasis-inducing pathways regulate histone chaperones to reduce canonical histone incorporation into chromatin, triggering deposition of H3.3 variant at the promoters of poor-prognosis genes and metastasis-inducing transcription factors. This specific incorporation of H3.3 into chromatin is both necessary and sufficient for the induction of aggressive traits that allow for metastasis formation. Together, our data clearly show incorporation of histone variant H3.3 into chromatin as a major regulator of cell fate during tumorigenesis, and histone chaperones as valuable therapeutic targets for invasive carcinomas.
中文翻译:

将组蛋白H3变体动态整合到染色质中对于获得侵略性特征和转移性定植至关重要。
转移是癌症死亡的主要原因。染色质重塑为驱动转移所必需的细胞重编程提供了基础。但是,对于这种重塑的性质及其调节知之甚少。在这里,我们显示转移诱导途径调节组蛋白伴侣以减少规范组蛋白掺入染色质中,触发预后不良基因和转移诱导转录因子启动子处的H3.3变体沉积。H3.3向染色质的这种特殊掺入对于诱导侵袭性特征(允许转移形成)既是必要的又是充分的。总之,我们的数据清楚地表明,在肿瘤发生过程中,组蛋白变体H3.3掺入了染色质中作为细胞命运的主要调节剂,
更新日期:2019-11-09
中文翻译:

将组蛋白H3变体动态整合到染色质中对于获得侵略性特征和转移性定植至关重要。
转移是癌症死亡的主要原因。染色质重塑为驱动转移所必需的细胞重编程提供了基础。但是,对于这种重塑的性质及其调节知之甚少。在这里,我们显示转移诱导途径调节组蛋白伴侣以减少规范组蛋白掺入染色质中,触发预后不良基因和转移诱导转录因子启动子处的H3.3变体沉积。H3.3向染色质的这种特殊掺入对于诱导侵袭性特征(允许转移形成)既是必要的又是充分的。总之,我们的数据清楚地表明,在肿瘤发生过程中,组蛋白变体H3.3掺入了染色质中作为细胞命运的主要调节剂,











































 京公网安备 11010802027423号
京公网安备 11010802027423号