当前位置:
X-MOL 学术
›
JAMA Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial.
JAMA Oncology ( IF 22.5 ) Pub Date : 2019-09-29 , DOI: 10.1001/jamaoncol.2019.4782 George W Sledge 1 , Masakazu Toi 2 , Patrick Neven 3 , Joohyuk Sohn 4 , Kenichi Inoue 5 , Xavier Pivot 6 , Olga Burdaeva 7 , Meena Okera 8 , Norikazu Masuda 9 , Peter A Kaufman 10 , Han Koh 11 , Eva-Maria Grischke 12 , PierFranco Conte 13 , Yi Lu 14 , Susana Barriga 15 , Karla Hurt 14 , Martin Frenzel 14 , Stephen Johnston 16 , Antonio Llombart-Cussac 17
JAMA Oncology ( IF 22.5 ) Pub Date : 2019-09-29 , DOI: 10.1001/jamaoncol.2019.4782 George W Sledge 1 , Masakazu Toi 2 , Patrick Neven 3 , Joohyuk Sohn 4 , Kenichi Inoue 5 , Xavier Pivot 6 , Olga Burdaeva 7 , Meena Okera 8 , Norikazu Masuda 9 , Peter A Kaufman 10 , Han Koh 11 , Eva-Maria Grischke 12 , PierFranco Conte 13 , Yi Lu 14 , Susana Barriga 15 , Karla Hurt 14 , Martin Frenzel 14 , Stephen Johnston 16 , Antonio Llombart-Cussac 17
Affiliation
|
Importance
Statistically significant overall survival (OS) benefits of CDK4 and CDK6 inhibitors in combination with fulvestrant for hormone receptor (HR)-positive, ERBB2 (formerly HER2)-negative advanced breast cancer (ABC) in patients regardless of menopausal status after prior endocrine therapy (ET) has not yet been demonstrated.
Objective
To compare the effect of abemaciclib plus fulvestrant vs placebo plus fulvestrant on OS at the prespecified interim of MONARCH 2 (338 events) in patients with HR-positive, ERBB2-negative advanced breast cancer that progressed during prior ET.
Design, Setting, and Participants
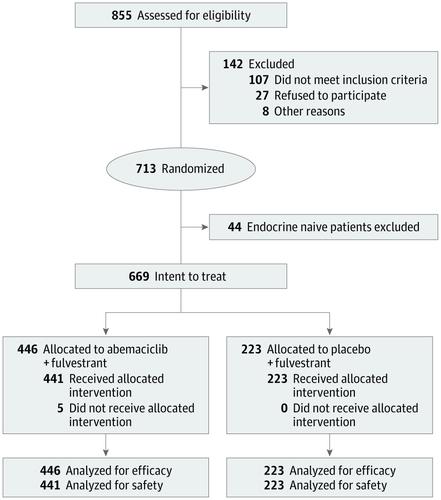
MONARCH 2 was a global, randomized, placebo-controlled, double-blind phase 3 trial of abemaciclib plus fulvestrant vs placebo plus fulvestrant for treatment of premenopausal or perimenopausal women (with ovarian suppression) and postmenopausal women with HR-positive, ERBB2-negative ABC that progressed during ET. Patients were enrolled between August 7, 2014, and December 29, 2015. Analyses for this report were conducted at the time of database lock on June 20, 2019.
Interventions
Patients were randomized 2:1 to receive abemaciclib or placebo, 150 mg, every 12 hours on a continuous schedule plus fulvestrant, 500 mg, per label. Randomization was stratified based on site of metastasis (visceral, bone only, or other) and resistance to prior ET (primary vs secondary).
Main Outcomes and Measures
The primary end point was investigator-assessed progression-free survival. Overall survival was a gated key secondary end point. The boundary P value for the interim analysis was .02.
Results
Of 669 women enrolled, 446 (median [range] age, 59 [32-91] years) were randomized to the abemaciclib plus fulvestrant arm and 223 (median [range] age, 62 [32-87] years) were randomized to the placebo plus fulvestrant arm. At the prespecified interim, 338 deaths (77% of the planned 441 at the final analysis) were observed in the intent-to-treat population, with a median OS of 46.7 months for abemaciclib plus fulvestrant and 37.3 months for placebo plus fulvestrant (hazard ratio [HR], 0.757; 95% CI, 0.606-0.945; P = .01). Improvement in OS was consistent across all stratification factors. Among stratification factors, more pronounced effects were observed in patients with visceral disease (HR, 0.675; 95% CI, 0.511-0.891) and primary resistance to prior ET (HR, 0.686; 95% CI, 0.451-1.043). Time to second disease progression (median, 23.1 months vs 20.6 months), time to chemotherapy (median, 50.2 months vs 22.1 months), and chemotherapy-free survival (median, 25.5 months vs 18.2 months) were also statistically significantly improved in the abemaciclib arm vs placebo arm. No new safety signals were observed for abemaciclib.
Conclusions and Relevance
Treatment with abemaciclib plus fulvestrant resulted in a statistically significant and clinically meaningful median OS improvement of 9.4 months for patients with HR-positive, ERBB2-negative ABC who progressed after prior ET regardless of menopausal status. Abemaciclib substantially delayed the receipt of subsequent chemotherapy.
Trial Registration
ClinicalTrials.gov Identifier: NCT02107703.
中文翻译:

Abemaciclib 加氟维司群对内分泌治疗进展的激素受体阳性、ERBB2 阴性乳腺癌总体生存的影响-MONARCH 2:一项随机临床试验。
CDK4 和 CDK6 抑制剂联合氟维司群治疗激素受体 (HR) 阳性、ERBB2(原 HER2)阴性晚期乳腺癌 (ABC) 患者无论在先前内分泌治疗后的绝经状态如何,均具有统计学意义的显着总生存期 (OS) 益处(ET) 尚未得到证实。目的比较 abemaciclib 加氟维司群与安慰剂加氟维司群在 MONARCH 2 的预定中期(338 起事件)对既往 ET 期间进展的 HR 阳性、ERBB2 阴性晚期乳腺癌患者 OS 的影响。设计、设置和参与者 MONARCH 2 是一项全球性、随机、安慰剂对照、abemaciclib 加氟维司群对比安慰剂加氟维司群的双盲 3 期试验,用于治疗在 ET 期间进展的 HR 阳性、ERBB2 阴性 ABC 的绝经前或围绝经期妇女(卵巢抑制)和绝经后妇女。患者于 2014 年 8 月 7 日至 2015 年 12 月 29 日期间入组。本报告的分析是在 2019 年 6 月 20 日数据库锁定时进行的。干预 患者按 2:1 随机分配接受 abemaciclib 或安慰剂,每次 150 mg连续 12 小时加氟维司群,每个标签 500 毫克。根据转移部位(内脏、仅骨或其他)和对先前 ET 的抵抗(原发性与继发性)对随机化进行分层。主要结果和措施 主要终点是研究者评估的无进展生存期。总生存期是一个封闭的关键次要终点。中期分析的边界 P 值为 0.02。结果 入组的 669 名女性中,446 名(中位 [范围] 年龄,59 [32-91] 岁)被随机分配至 abemaciclib 加氟维司群组,223 名(中位 [范围] 年龄,62 [32-87] 岁)被随机分配至安慰剂加氟维司群组。在预定的中期,在意向治疗人群中观察到 338 例死亡(最终分析时计划的 441 例的 77%),abemaciclib 加氟维司群的中位 OS 为 46.7 个月,安慰剂加氟维司群为 37.3 个月(危险比率 [HR],0.757;95% CI,0.606-0.945;P = .01)。OS 的改善在所有分层因素中都是一致的。在分层因素中,在内脏疾病患者中观察到更显着的影响(HR,0.675;95% CI,0.511-0。891)和对先前 ET 的主要抵抗(HR,0.686;95% CI,0.451-1.043)。在 abemaciclib 中,到第二次疾病进展的时间(中位数,23.1 个月 vs 20.6 个月)、化疗时间(中位数,50.2 个月 vs 22.1 个月)和无化疗生存期(中位数,25.5 个月 vs 18.2 个月)也有统计学意义的改善手臂与安慰剂手臂。没有观察到 abemaciclib 的新安全信号。结论和相关性 abemaciclib 加氟维司群治疗使 HR 阳性、ERBB2 阴性 ABC 患者的中位 OS 改善了 9.4 个月,这些患者在既往 ET 后进展,无论绝经状态如何。Abemaciclib 大大延迟了后续化疗的接受。试验注册 ClinicalTrials.gov 标识符:NCT02107703。
更新日期:2020-01-09
中文翻译:

Abemaciclib 加氟维司群对内分泌治疗进展的激素受体阳性、ERBB2 阴性乳腺癌总体生存的影响-MONARCH 2:一项随机临床试验。
CDK4 和 CDK6 抑制剂联合氟维司群治疗激素受体 (HR) 阳性、ERBB2(原 HER2)阴性晚期乳腺癌 (ABC) 患者无论在先前内分泌治疗后的绝经状态如何,均具有统计学意义的显着总生存期 (OS) 益处(ET) 尚未得到证实。目的比较 abemaciclib 加氟维司群与安慰剂加氟维司群在 MONARCH 2 的预定中期(338 起事件)对既往 ET 期间进展的 HR 阳性、ERBB2 阴性晚期乳腺癌患者 OS 的影响。设计、设置和参与者 MONARCH 2 是一项全球性、随机、安慰剂对照、abemaciclib 加氟维司群对比安慰剂加氟维司群的双盲 3 期试验,用于治疗在 ET 期间进展的 HR 阳性、ERBB2 阴性 ABC 的绝经前或围绝经期妇女(卵巢抑制)和绝经后妇女。患者于 2014 年 8 月 7 日至 2015 年 12 月 29 日期间入组。本报告的分析是在 2019 年 6 月 20 日数据库锁定时进行的。干预 患者按 2:1 随机分配接受 abemaciclib 或安慰剂,每次 150 mg连续 12 小时加氟维司群,每个标签 500 毫克。根据转移部位(内脏、仅骨或其他)和对先前 ET 的抵抗(原发性与继发性)对随机化进行分层。主要结果和措施 主要终点是研究者评估的无进展生存期。总生存期是一个封闭的关键次要终点。中期分析的边界 P 值为 0.02。结果 入组的 669 名女性中,446 名(中位 [范围] 年龄,59 [32-91] 岁)被随机分配至 abemaciclib 加氟维司群组,223 名(中位 [范围] 年龄,62 [32-87] 岁)被随机分配至安慰剂加氟维司群组。在预定的中期,在意向治疗人群中观察到 338 例死亡(最终分析时计划的 441 例的 77%),abemaciclib 加氟维司群的中位 OS 为 46.7 个月,安慰剂加氟维司群为 37.3 个月(危险比率 [HR],0.757;95% CI,0.606-0.945;P = .01)。OS 的改善在所有分层因素中都是一致的。在分层因素中,在内脏疾病患者中观察到更显着的影响(HR,0.675;95% CI,0.511-0。891)和对先前 ET 的主要抵抗(HR,0.686;95% CI,0.451-1.043)。在 abemaciclib 中,到第二次疾病进展的时间(中位数,23.1 个月 vs 20.6 个月)、化疗时间(中位数,50.2 个月 vs 22.1 个月)和无化疗生存期(中位数,25.5 个月 vs 18.2 个月)也有统计学意义的改善手臂与安慰剂手臂。没有观察到 abemaciclib 的新安全信号。结论和相关性 abemaciclib 加氟维司群治疗使 HR 阳性、ERBB2 阴性 ABC 患者的中位 OS 改善了 9.4 个月,这些患者在既往 ET 后进展,无论绝经状态如何。Abemaciclib 大大延迟了后续化疗的接受。试验注册 ClinicalTrials.gov 标识符:NCT02107703。











































 京公网安备 11010802027423号
京公网安备 11010802027423号