Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oral Lefamulin vs Moxifloxacin for Early Clinical Response Among Adults With Community-Acquired Bacterial Pneumonia
JAMA ( IF 63.1 ) Pub Date : 2019-11-05 , DOI: 10.1001/jama.2019.15468 Elizabeth Alexander 1 , Lisa Goldberg 1 , Anita F Das 2 , Gregory J Moran 3 , Christian Sandrock 4 , Leanne B Gasink 1 , Patricia Spera 1 , Carolyn Sweeney 1 , Susanne Paukner 5 , Wolfgang W Wicha 5 , Steven P Gelone 1 , Jennifer Schranz 1
JAMA ( IF 63.1 ) Pub Date : 2019-11-05 , DOI: 10.1001/jama.2019.15468 Elizabeth Alexander 1 , Lisa Goldberg 1 , Anita F Das 2 , Gregory J Moran 3 , Christian Sandrock 4 , Leanne B Gasink 1 , Patricia Spera 1 , Carolyn Sweeney 1 , Susanne Paukner 5 , Wolfgang W Wicha 5 , Steven P Gelone 1 , Jennifer Schranz 1
Affiliation
|
Importance
New antibacterials are needed to treat community-acquired bacterial pneumonia (CABP) because of growing antibacterial resistance and safety concerns with standard care. Objective
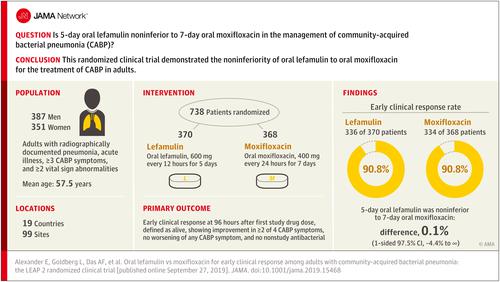
To evaluate the efficacy and adverse events of a 5-day oral lefamulin regimen in patients with CABP. Design, Setting, and Participants
A phase 3, noninferiority randomized clinical trial conducted at 99 sites in 19 countries that included adults aged 18 years or older with a Pneumonia Outcomes Research Team (PORT) risk class of II, III, or IV; radiographically documented pneumonia; acute illness; 3 or more CABP symptoms; and 2 or more vital sign abnormalities. The first patient visit was on August 30, 2016, and patients were followed up for 30 days; the final follow-up visit was on January 2, 2018. Interventions
Patients were randomized 1:1 to receive oral lefamulin (600 mg every 12 hours for 5 days; n = 370) or moxifloxacin (400 mg every 24 hours for 7 days; n = 368). Main Outcomes and Measures
The US Food and Drug Administration (FDA) primary end point was early clinical response at 96 hours (within a 24-hour window) after the first dose of either study drug in the intent-to-treat (ITT) population (all randomized patients). Responders were defined as alive, showing improvement in 2 or more of the 4 CABP symptoms, having no worsening of any CABP symptoms, and not receiving any nonstudy antibacterial drug for current CABP episode. The European Medicines Agency coprimary end points (FDA secondary end points) were investigator assessment of clinical response at test of cure (5-10 days after last dose) in the modified ITT population and in the clinically evaluable population. The noninferiority margin was 10% for early clinical response and investigator assessment of clinical response. Results
Among 738 randomized patients (mean age, 57.5 years; 351 women [47.6%]; 360 had a PORT risk class of III or IV [48.8%]), 707 (95.8%) completed the trial. Early clinical response rates were 90.8% with lefamulin and 90.8% with moxifloxacin (difference, 0.1% [1-sided 97.5% CI, -4.4% to ∞]). Rates of investigator assessment of clinical response success were 87.5% with lefamulin and 89.1% with moxifloxacin in the modified ITT population (difference, -1.6% [1-sided 97.5% CI, -6.3% to ∞]) and 89.7% and 93.6%, respectively, in the clinically evaluable population (difference, -3.9% [1-sided 97.5% CI, -8.2% to ∞]) at test of cure. The most frequently reported treatment-emergent adverse events were gastrointestinal (diarrhea: 45/368 [12.2%] in lefamulin group and 4/368 [1.1%] in moxifloxacin group; nausea: 19/368 [5.2%] in lefamulin group and 7/368 [1.9%] in moxifloxacin group). Conclusions and Relevance
Among patients with CABP, 5-day oral lefamulin was noninferior to 7-day oral moxifloxacin with respect to early clinical response at 96 hours after first dose. Trial Registrations
ClinicalTrials.gov Identifier: NCT02813694; European Clinical Trials Identifier: 2015-004782-92.
中文翻译:

口服来法莫林与莫西沙星对社区获得性细菌性肺炎成人早期临床反应的比较
重要性 由于抗菌药物耐药性不断增加以及标准护理的安全性问题,需要新的抗菌药物来治疗社区获得性细菌性肺炎 (CABP)。目的评价 5 天口服来法莫林方案治疗 CABP 的疗效和不良事件。设计、设置和参与者 在 19 个国家的 99 个地点进行的 3 期非劣效性随机临床试验,包括肺炎结果研究小组 (PORT) 风险等级为 II、III 或 IV 的 18 岁或以上成年人;放射线记录的肺炎;急性疾病;3 种或更多 CABP 症状;以及 2 种或更多生命体征异常。首次患者就诊时间为2016年8月30日,随访患者30天;最后一次随访于 2018 年 1 月 2 日进行。 干预措施 患者以 1:1 的比例随机接受口服来法莫林(每 12 小时 600 毫克,持续 5 天;n = 370)或莫西沙星(每 24 小时 400 毫克,持续 7 天;n = 370)。 n = 368)。主要结果和措施 美国食品和药物管理局 (FDA) 主要终点是意向治疗 (ITT) 人群首次服用任一研究药物后 96 小时(24 小时窗口内)的早期临床反应(所有随机分组的患者)。应答者被定义为存活,4 种 CABP 症状中的 2 种或更多症状有所改善,任何 CABP 症状没有恶化,并且没有针对当前 CABP 发作接受任何非研究抗菌药物。欧洲药品管理局共同主要终点(FDA 次要终点)是研究者对改良 ITT 人群和临床可评估人群治愈试验(最后一次给药后 5-10 天)临床反应的评估。早期临床反应和研究者对临床反应的评估的非劣效性界限为 10%。结果 在 738 名随机患者(平均年龄 57.5 岁;351 名女性 [47.6%];360 名 PORT 风险级别为 III 或 IV [48.8%])中,707 名 (95.8%) 完成了试验。来法莫林的早期临床缓解率为 90.8%,莫西沙星的早期临床缓解率为 90.8%(差异为 0.1% [单侧 97.5% CI,-4.4% 至 ∞])。在改良 ITT 人群中,研究者评估临床缓解成功率,来法莫林为 87.5%,莫西沙星为 89.1%(差异,-1.6% [单侧 97.5% CI,-6.3% 至 ∞])以及 89.7% 和 93.6%分别在治愈测试时的临床可评估人群中(差异,-3.9% [单侧 97.5% CI,-8.2% 至 ∞])。最常报告的治疗中出现的不良事件是胃肠道(腹泻:来法莫林组为 45/368 [12.2%],莫西沙星组为 4/368 [1.1%];恶心:来法莫林组为 19/368 [5.2%],莫西沙星组为 7 /368 [1.9%](莫西沙星组)。结论和相关性 在 CABP 患者中,就首次给药后 96 小时的早期临床反应而言,5 天口服来法莫林不劣于 7 天口服莫西沙星。试验注册 ClinicalTrials.gov 标识符:NCT02813694;欧洲临床试验标识符:2015-004782-92。
更新日期:2019-11-05
中文翻译:

口服来法莫林与莫西沙星对社区获得性细菌性肺炎成人早期临床反应的比较
重要性 由于抗菌药物耐药性不断增加以及标准护理的安全性问题,需要新的抗菌药物来治疗社区获得性细菌性肺炎 (CABP)。目的评价 5 天口服来法莫林方案治疗 CABP 的疗效和不良事件。设计、设置和参与者 在 19 个国家的 99 个地点进行的 3 期非劣效性随机临床试验,包括肺炎结果研究小组 (PORT) 风险等级为 II、III 或 IV 的 18 岁或以上成年人;放射线记录的肺炎;急性疾病;3 种或更多 CABP 症状;以及 2 种或更多生命体征异常。首次患者就诊时间为2016年8月30日,随访患者30天;最后一次随访于 2018 年 1 月 2 日进行。 干预措施 患者以 1:1 的比例随机接受口服来法莫林(每 12 小时 600 毫克,持续 5 天;n = 370)或莫西沙星(每 24 小时 400 毫克,持续 7 天;n = 370)。 n = 368)。主要结果和措施 美国食品和药物管理局 (FDA) 主要终点是意向治疗 (ITT) 人群首次服用任一研究药物后 96 小时(24 小时窗口内)的早期临床反应(所有随机分组的患者)。应答者被定义为存活,4 种 CABP 症状中的 2 种或更多症状有所改善,任何 CABP 症状没有恶化,并且没有针对当前 CABP 发作接受任何非研究抗菌药物。欧洲药品管理局共同主要终点(FDA 次要终点)是研究者对改良 ITT 人群和临床可评估人群治愈试验(最后一次给药后 5-10 天)临床反应的评估。早期临床反应和研究者对临床反应的评估的非劣效性界限为 10%。结果 在 738 名随机患者(平均年龄 57.5 岁;351 名女性 [47.6%];360 名 PORT 风险级别为 III 或 IV [48.8%])中,707 名 (95.8%) 完成了试验。来法莫林的早期临床缓解率为 90.8%,莫西沙星的早期临床缓解率为 90.8%(差异为 0.1% [单侧 97.5% CI,-4.4% 至 ∞])。在改良 ITT 人群中,研究者评估临床缓解成功率,来法莫林为 87.5%,莫西沙星为 89.1%(差异,-1.6% [单侧 97.5% CI,-6.3% 至 ∞])以及 89.7% 和 93.6%分别在治愈测试时的临床可评估人群中(差异,-3.9% [单侧 97.5% CI,-8.2% 至 ∞])。最常报告的治疗中出现的不良事件是胃肠道(腹泻:来法莫林组为 45/368 [12.2%],莫西沙星组为 4/368 [1.1%];恶心:来法莫林组为 19/368 [5.2%],莫西沙星组为 7 /368 [1.9%](莫西沙星组)。结论和相关性 在 CABP 患者中,就首次给药后 96 小时的早期临床反应而言,5 天口服来法莫林不劣于 7 天口服莫西沙星。试验注册 ClinicalTrials.gov 标识符:NCT02813694;欧洲临床试验标识符:2015-004782-92。











































 京公网安备 11010802027423号
京公网安备 11010802027423号