当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of modifiable photo-responsive polypeptides bearing allyloxyazobenzene side-chains†
Polymer Chemistry ( IF 4.1 ) Pub Date : 2019-09-27 , DOI: 10.1039/c9py01106d Wei Xiong 1, 2, 3, 4, 5 , Chong Zhang 1, 2, 3, 4, 5 , Xiaolin Lyu 1, 2, 3, 4, 5 , Hantao Zhou 1, 2, 3, 4, 5 , Wenying Chang 1, 2, 3, 4, 5 , Yu Bo 1, 2, 3, 4, 5 , Erqiang Chen 1, 2, 3, 4, 5 , Zhihao Shen 1, 2, 3, 4, 5 , Hua Lu 1, 2, 3, 4, 5
Polymer Chemistry ( IF 4.1 ) Pub Date : 2019-09-27 , DOI: 10.1039/c9py01106d Wei Xiong 1, 2, 3, 4, 5 , Chong Zhang 1, 2, 3, 4, 5 , Xiaolin Lyu 1, 2, 3, 4, 5 , Hantao Zhou 1, 2, 3, 4, 5 , Wenying Chang 1, 2, 3, 4, 5 , Yu Bo 1, 2, 3, 4, 5 , Erqiang Chen 1, 2, 3, 4, 5 , Zhihao Shen 1, 2, 3, 4, 5 , Hua Lu 1, 2, 3, 4, 5
Affiliation
|
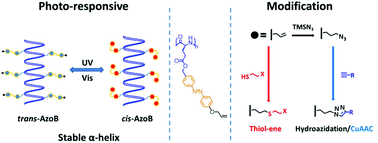
Synthetic polypeptides are important protein mimics due to their similar primary and secondary structures. Previously, the properties of polypeptides can be regulated by either introducing N-carboxyanhydride (NCA) stimuli-responsive moieties directly or modifiable groups for post-polymerization functionalization. Here we combine the two approaches and report the synthesis of photo-responsive and modifiable helical poly(L-glutamate) bearing an azobenzene (Azo) and an alkene (“ene”) group in the side chain, namely P(AzoEne-Glu)s. The polypeptides, prepared by the controlled ring-opening polymerization of a novel monomer AzoEne-GluNCA, undergo rapid and high yield UV-triggered trans–cis isomerization of the Azo moiety. However, this change in the primary structure does not lead to interruption of the helical secondary structure. The rigid helical main-chain and pendent Azo mesogen together endow P(AzoEne-Glu)s with liquid crystalline properties. Moreover, the “ene” group of P(AzoEne-Glu)s can be modified with thiols via a UV-mediated thiol–ene reaction, or quantitatively transformed to azide via a novel anti-Markovnikov hydroazidation approach. The newly generated azido-containing polymer can be further converted by click-type cycloaddition reactions. This work may streamline the rapid synthesis of diverse functional and stimuli-responsive polypeptides that are potentially useful for self-assembly and liquid crystalline materials.
中文翻译:

带有烯丙氧基偶氮苯侧链的可修饰光反应性多肽的合成†
合成多肽由于其相似的一级和二级结构而成为重要的蛋白质模拟物。以前,可以通过直接引入N-羧基酸酐(NCA)刺激响应部分或用于聚合后官能化的可修饰基团来调节多肽的特性。在这里,我们结合这两种方法,并报告了在侧链上带有偶氮苯(Azo)和烯基(“ ene”)的光响应性和可改性螺旋聚(L-谷氨酸)的合成,即P(AzoEne-Glu) s。多肽,由一种新颖的单体AzoEne-GluNCA的受控开环聚合制备的,经历快速和高产率的UV触发的反式-顺式偶氮部分的异构化。但是,一级结构的这种变化不会导致螺旋二级结构的中断。刚性的螺旋主链和悬垂的偶氮液晶元共同赋予P(AzoEne-Glu)液晶特性。此外,P(偶氮烯-Glu)的“烯”基团可通过紫外线介导的硫醇-烯反应用硫醇修饰,或通过新型的反马尔可夫尼科夫加氢叠氮方法定量转化为叠氮化物。新产生的含叠氮基的聚合物可以通过点击型环加成反应进一步转化。这项工作可以简化各种功能和刺激响应多肽的快速合成,这些多肽可能对自组装和液晶材料有用。
更新日期:2020-01-02
中文翻译:

带有烯丙氧基偶氮苯侧链的可修饰光反应性多肽的合成†
合成多肽由于其相似的一级和二级结构而成为重要的蛋白质模拟物。以前,可以通过直接引入N-羧基酸酐(NCA)刺激响应部分或用于聚合后官能化的可修饰基团来调节多肽的特性。在这里,我们结合这两种方法,并报告了在侧链上带有偶氮苯(Azo)和烯基(“ ene”)的光响应性和可改性螺旋聚(L-谷氨酸)的合成,即P(AzoEne-Glu) s。多肽,由一种新颖的单体AzoEne-GluNCA的受控开环聚合制备的,经历快速和高产率的UV触发的反式-顺式偶氮部分的异构化。但是,一级结构的这种变化不会导致螺旋二级结构的中断。刚性的螺旋主链和悬垂的偶氮液晶元共同赋予P(AzoEne-Glu)液晶特性。此外,P(偶氮烯-Glu)的“烯”基团可通过紫外线介导的硫醇-烯反应用硫醇修饰,或通过新型的反马尔可夫尼科夫加氢叠氮方法定量转化为叠氮化物。新产生的含叠氮基的聚合物可以通过点击型环加成反应进一步转化。这项工作可以简化各种功能和刺激响应多肽的快速合成,这些多肽可能对自组装和液晶材料有用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号