Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Translational offsetting as a mode of estrogen receptor α-dependent regulation of gene expression.
The EMBO Journal ( IF 9.4 ) Pub Date : 2019-09-26 , DOI: 10.15252/embj.2018101323 Julie Lorent 1 , Eric P Kusnadi 2, 3, 4 , Vincent van Hoef 1 , Richard J Rebello 2, 3 , Matthew Leibovitch 5 , Johannes Ristau 1 , Shan Chen 1 , Mitchell G Lawrence 2, 3, 4 , Krzysztof J Szkop 1 , Baila Samreen 1 , Preetika Balanathan 3 , Francesca Rapino 6 , Pierre Close 6 , Patricia Bukczynska 2 , Karin Scharmann 7, 8 , Itsuhiro Takizawa 3 , Gail P Risbridger 2, 3, 4 , Luke A Selth 9 , Sebastian A Leidel 7, 8, 10 , Qishan Lin 11 , Ivan Topisirovic 5 , Ola Larsson 1 , Luc Furic 2, 3, 4
The EMBO Journal ( IF 9.4 ) Pub Date : 2019-09-26 , DOI: 10.15252/embj.2018101323 Julie Lorent 1 , Eric P Kusnadi 2, 3, 4 , Vincent van Hoef 1 , Richard J Rebello 2, 3 , Matthew Leibovitch 5 , Johannes Ristau 1 , Shan Chen 1 , Mitchell G Lawrence 2, 3, 4 , Krzysztof J Szkop 1 , Baila Samreen 1 , Preetika Balanathan 3 , Francesca Rapino 6 , Pierre Close 6 , Patricia Bukczynska 2 , Karin Scharmann 7, 8 , Itsuhiro Takizawa 3 , Gail P Risbridger 2, 3, 4 , Luke A Selth 9 , Sebastian A Leidel 7, 8, 10 , Qishan Lin 11 , Ivan Topisirovic 5 , Ola Larsson 1 , Luc Furic 2, 3, 4
Affiliation
|
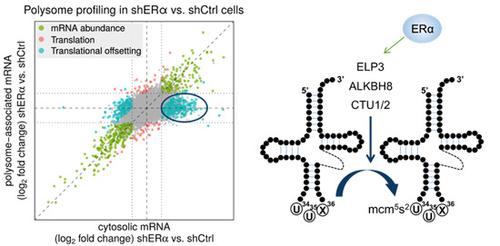
Estrogen receptor alpha (ERα) activity is associated with increased cancer cell proliferation. Studies aiming to understand the impact of ERα on cancer-associated phenotypes have largely been limited to its transcriptional activity. Herein, we demonstrate that ERα coordinates its transcriptional output with selective modulation of mRNA translation. Importantly, translational perturbations caused by depletion of ERα largely manifest as "translational offsetting" of the transcriptome, whereby amounts of translated mRNAs and corresponding protein levels are maintained constant despite changes in mRNA abundance. Transcripts whose levels, but not polysome association, are reduced following ERα depletion lack features which limit translation efficiency including structured 5'UTRs and miRNA target sites. In contrast, mRNAs induced upon ERα depletion whose polysome association remains unaltered are enriched in codons requiring U34-modified tRNAs for efficient decoding. Consistently, ERα regulates levels of U34-modifying enzymes and thereby controls levels of U34-modified tRNAs. These findings unravel a hitherto unprecedented mechanism of ERα-dependent orchestration of transcriptional and translational programs that may be a pervasive mechanism of proteome maintenance in hormone-dependent cancers.
中文翻译:

翻译抵消作为雌激素受体α依赖性基因表达调节的一种模式。
雌激素受体α (ERα) 活性与癌细胞增殖增加相关。旨在了解 ERα 对癌症相关表型影响的研究很大程度上仅限于其转录活性。在此,我们证明 ERα 通过选择性调节 mRNA 翻译来协调其转录输出。重要的是,由 ERα 耗尽引起的翻译扰动很大程度上表现为转录组的“翻译偏移”,即尽管 mRNA 丰度发生变化,翻译的 mRNA 量和相应的蛋白质水平仍保持恒定。 ERα 耗尽后,其水平(而非多核糖体关联)降低的转录物缺乏限制翻译效率的特征,包括结构化 5'UTR 和 miRNA 靶位点。相比之下,ERα 耗尽后诱导的 mRNA(其多核糖体关联保持不变)富含密码子,需要 U34 修饰的 tRNA 才能有效解码。一致地,ERα 调节 U34 修饰酶的水平,从而控制 U34 修饰 tRNA 的水平。这些发现揭示了迄今为止前所未有的 ERα 依赖性转录和翻译程序编排机制,这可能是激素依赖性癌症中蛋白质组维持的普遍机制。
更新日期:2019-12-02
中文翻译:

翻译抵消作为雌激素受体α依赖性基因表达调节的一种模式。
雌激素受体α (ERα) 活性与癌细胞增殖增加相关。旨在了解 ERα 对癌症相关表型影响的研究很大程度上仅限于其转录活性。在此,我们证明 ERα 通过选择性调节 mRNA 翻译来协调其转录输出。重要的是,由 ERα 耗尽引起的翻译扰动很大程度上表现为转录组的“翻译偏移”,即尽管 mRNA 丰度发生变化,翻译的 mRNA 量和相应的蛋白质水平仍保持恒定。 ERα 耗尽后,其水平(而非多核糖体关联)降低的转录物缺乏限制翻译效率的特征,包括结构化 5'UTR 和 miRNA 靶位点。相比之下,ERα 耗尽后诱导的 mRNA(其多核糖体关联保持不变)富含密码子,需要 U34 修饰的 tRNA 才能有效解码。一致地,ERα 调节 U34 修饰酶的水平,从而控制 U34 修饰 tRNA 的水平。这些发现揭示了迄今为止前所未有的 ERα 依赖性转录和翻译程序编排机制,这可能是激素依赖性癌症中蛋白质组维持的普遍机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号