npj Digital Medicine ( IF 12.4 ) Pub Date : 2019-09-25 , DOI: 10.1038/s41746-019-0168-z Alison Callahan 1 , Jason A Fries 1, 2 , Christopher Ré 2 , James I Huddleston 3 , Nicholas J Giori 3, 4 , Scott Delp 5 , Nigam H Shah 1
|
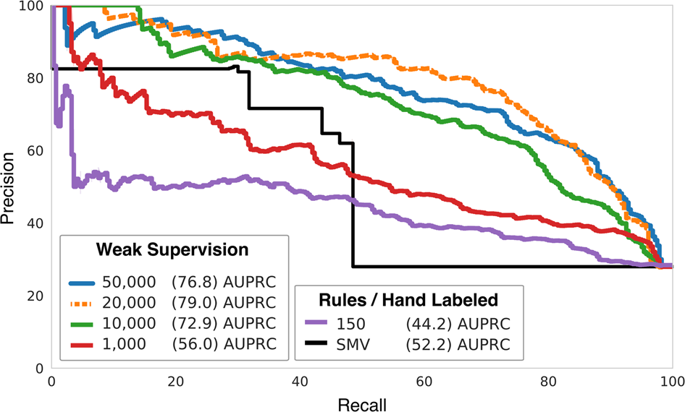
Post-market medical device surveillance is a challenge facing manufacturers, regulatory agencies, and health care providers. Electronic health records are valuable sources of real-world evidence for assessing device safety and tracking device-related patient outcomes over time. However, distilling this evidence remains challenging, as information is fractured across clinical notes and structured records. Modern machine learning methods for machine reading promise to unlock increasingly complex information from text, but face barriers due to their reliance on large and expensive hand-labeled training sets. To address these challenges, we developed and validated state-of-the-art deep learning methods that identify patient outcomes from clinical notes without requiring hand-labeled training data. Using hip replacements—one of the most common implantable devices—as a test case, our methods accurately extracted implant details and reports of complications and pain from electronic health records with up to 96.3% precision, 98.5% recall, and 97.4% F1, improved classification performance by 12.8–53.9% over rule-based methods, and detected over six times as many complication events compared to using structured data alone. Using these additional events to assess complication-free survivorship of different implant systems, we found significant variation between implants, including for risk of revision surgery, which could not be detected using coded data alone. Patients with revision surgeries had more hip pain mentions in the post-hip replacement, pre-revision period compared to patients with no evidence of revision surgery (mean hip pain mentions 4.97 vs. 3.23; t = 5.14; p < 0.001). Some implant models were associated with higher or lower rates of hip pain mentions. Our methods complement existing surveillance mechanisms by requiring orders of magnitude less hand-labeled training data, offering a scalable solution for national medical device surveillance using electronic health records.
中文翻译:

通过电子健康记录进行医疗设备监控
上市后医疗器械监管是制造商、监管机构和医疗保健提供者面临的挑战。电子健康记录是真实世界证据的宝贵来源,可用于评估设备安全性和跟踪设备相关患者随时间的结果。然而,提取这些证据仍然具有挑战性,因为信息在临床记录和结构化记录中是分散的。用于机器阅读的现代机器学习方法有望从文本中解锁日益复杂的信息,但由于依赖于大型且昂贵的手工标记训练集而面临障碍。为了应对这些挑战,我们开发并验证了最先进的深度学习方法,可以从临床记录中识别患者的结果,而无需手工标记的训练数据。使用髋关节置换术(最常见的植入设备之一)作为测试案例,我们的方法从电子健康记录中准确提取植入细节以及并发症和疼痛报告,精确度高达 96.3%,召回率高达 98.5%,F1 高达 97.4%,改进与基于规则的方法相比,分类性能提高了 12.8-53.9%,检测到的并发症事件数量是单独使用结构化数据的六倍多。利用这些额外的事件来评估不同种植体系统的无并发症生存率,我们发现种植体之间存在显着差异,包括翻修手术的风险,而仅使用编码数据无法检测到这种差异。与没有翻修手术证据的患者相比,进行翻修手术的患者在髋关节置换后、翻修前期间提及的髋部疼痛更多(平均提及髋部疼痛为 4.97 vs. 3.23;t = 5.14; p < 0.001)。一些植入物模型与较高或较低的髋部疼痛提及率相关。 我们的方法通过减少数量级的手工标记训练数据来补充现有的监测机制,为使用电子健康记录的国家医疗器械监测提供可扩展的解决方案。











































 京公网安备 11010802027423号
京公网安备 11010802027423号