Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2019-09-25 , DOI: 10.1016/j.jfluchem.2019.109387 Keun Sam Jang , Sung-Sik Lee , Young-Ho Oh , Sang Hee Lee , Sang Eun Kim , Dong Wook Kim , Byung Chul Lee , Sungyul Lee , David M. Raffel
|
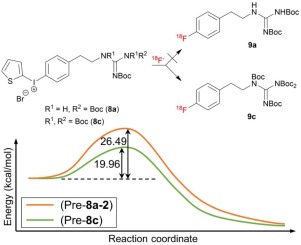
We analyze the effects of protecting group(s) on the efficiency of aromatic 18F-labeling of guanidine-containing radiopharmaceuticals by quantum chemical methods. We elucidate the origin of experimental observations: The fully protected N,N’,N’’,N’’-tetrakis-Boc guanidine group exhibits remarkably enhanced reactivity and improved selectivity in contrast to both N,N’-bis-Boc protected guanidine and its isomer in the absence of hydrogen bonding with fluoride ion. We find that this very intriguing observation of reaction rates highly depending on the position of protection by –Boc is due to the effects of strong Coulombic interactions among the ionic species, which allow an optimal position (just above the electropositive carbon and the leaving group) of F− in the reaction involving the fully protected N,N’,N’’,N’’-tetrakis-Boc guanidine group.
中文翻译:

通过监测保护基来控制胍基二碘鎓盐对18 F-标签的反应性和选择性:实验和理论
我们通过量子化学方法分析了保护基团对含胍基放射性药物芳香18 F标记效率的影响。我们阐明实验观察的起源:完全保护的Ñ,Ñ ”,Ñ ‘’,Ñ ‘’ -四叔丁氧羰基胍表现出显着增强对比度的反应性和改进的选择性两者Ñ,Ñ'-bis-Boc在没有氢键与氟离子结合的情况下保护了胍及其异构体。我们发现,这种非常有趣的反应速率观察结果很大程度上取决于–Boc的保护位置,这归因于离子种类之间强烈的库仑相互作用,从而产生了最佳位置(正电碳和离去基团上方)的F -在反应涉及完全保护ñ,ñ ”,ñ ‘’,ñ ‘’ -四-的Boc胍基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号