Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2019-09-25 , DOI: 10.1016/j.jfluchem.2019.109389 Reza Ranjbar-Karimi , Alireza Poorfreidoni
|
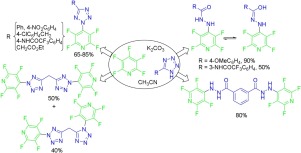
Reactivity of some 5-substituted tetrazoles with pentafluoropyridine under basic conditions in acetonitrile was investigated. The reaction of pentafluoropyridine with tetrazoles regioselectively occurs at the 4-position of pyridine ring by N2 in the tetrazole ring. Tetrazoles with substituents at the position 5 such as Ph, 4-NO2C6H4, 4-CF3CONHC6H4, 4-ClC6H4CH2, EtOOCCH2, and 5-tetrazolyl−CH2 gave products containing unchanged tetrazole moiety. In contrast, tetrazole with substituents at the position 5 such as 3-(5-tetrazolyl)C6H4, 4-MeOC6H4, and 3-CF3CONHC6H4, gave hydrazide products via degradation of tetrazole ring.
中文翻译:

调查5取代的四唑对五氟吡啶的反应性
在碱性条件下,研究了一些5-取代的四唑与五氟吡啶在乙腈中的反应性。五氟吡啶与四唑的区域选择性地在吡啶环的4-位被四唑环中的N 2发生。用的取代基的四唑在位置5,如pH值,4-NO 2 C ^ 6 ħ 4,4-CF 3 CONHC 6 H ^ 4,4-CLC 6 ħ 4 CH 2,EtOOCCH 2,和5-四唑基-CH 2,得到的产品含有未改变的四唑部分。相反,在位置5具有取代基的四唑,例如3-(5-四唑基)C 6 H4、4 -MeOC 6 H 4和3-CF 3 CONHC 6 H 4通过四唑环的降解得到酰肼产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号