当前位置:
X-MOL 学术
›
Dent. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel nanotechnology and near-infrared photodynamic therapy to kill periodontitis-related biofilm pathogens and protect the periodontium.
Dental Materials ( IF 4.6 ) Pub Date : 2019-09-21 , DOI: 10.1016/j.dental.2019.08.115 Manlin Qi 1 , Xue Li 1 , Xiaolin Sun 1 , Chunyan Li 1 , Franklin R Tay 2 , Michael D Weir 3 , Biao Dong 4 , Yanmin Zhou 1 , Lin Wang 1 , Hockin H K Xu 5
Dental Materials ( IF 4.6 ) Pub Date : 2019-09-21 , DOI: 10.1016/j.dental.2019.08.115 Manlin Qi 1 , Xue Li 1 , Xiaolin Sun 1 , Chunyan Li 1 , Franklin R Tay 2 , Michael D Weir 3 , Biao Dong 4 , Yanmin Zhou 1 , Lin Wang 1 , Hockin H K Xu 5
Affiliation
|
OBJECTIVE
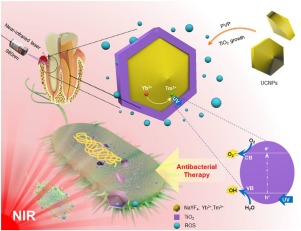
Periodontal tissue destruction and tooth loss are increasingly a worldwide problem as the population ages. Periodontitis is caused by bacterial infection and biofilm plaque buildup. Therefore, the objectives of this study were to: (1) develop a near-infrared light (NIR)-triggered core-shell nanostructure of upconversion nanoparticles and TiO2 (UCNPs@TiO2), and (2) investigate its inhibitory effects via antibacterial photodynamic therapy (aPDT) against periodontitis-related pathogens.
METHODS
The core β-NaYF4:Yb3+,Tm3+ were synthesized via thermal decomposition and further modified with the TiO2 shell via a hydrothermal method. The core-shell structure and the upconversion fluorescence-induced aPDT treatment via 980nm laser were studied. Three periodontitis-related pathogens Streptococcus sanguinis (S. sanguinis), Porphyromonas gingivalis (P. gingivalis) and Fusobacterium nucleatum (F. nucleatum) were investigated. The killing activity against planktonic bacteria was detected by a time-kill assay. Single species 4-day biofilms on dentin were tested by live/dead staining, colony-forming units (CFU), and metabolic activity.
RESULTS
The hexagonal shaped UCNPs@TiO2 had an average diameter of 39.7nm. UCNPs@TiO2 nanoparticles had positively charged (+12.4mV) surface and were biocompatible and non-cytotoxic. Under the excitation of NIR light (980nm), the core NaYF4:Yb3+,Tm3+ UCNPs could emit intense ultraviolet (UV) light, which further triggered the aPDT function of the shell TiO2 via energy transfer, thereby realizing the remarkable antibacterial effects against planktons and biofilms of periodontitis-associated pathogens. NIR-triggered UCNPs@TiO2 achieved much greater reduction in biofilms than control (p<0.05). Biofilm CFU was reduced by 3-4 orders of magnitude via NIR-triggered aPDT, which is significantly greater than that of negative control and commercial aPDT control groups. The killing efficacy of UCNPs@TiO2-based aPDT against the three species was ranked to be: S. sanguinis
中文翻译:

新型纳米技术和近红外光动力疗法可杀死与牙周炎相关的生物膜病原体并保护牙周病。
目的随着人口的老龄化,牙周组织的破坏和牙齿脱落越来越成为一个世界性的问题。牙周炎是由细菌感染和生物膜斑块堆积引起的。因此,本研究的目的是:(1)开发近红外(NIR)触发的上转换纳米颗粒和TiO2(UCNPs @ TiO2)的核-壳纳米结构,以及(2)通过抗菌光动力学研究其抑制作用牙周炎相关病原体的治疗(aPDT)。方法通过热分解合成β-NaYF4:Yb3 +,Tm3 +核,并通过水热法用TiO2壳进一步修饰。研究了980nm激光的核-壳结构和上转换荧光诱导的aPDT处理。三种与牙周炎相关的病原体链球菌血红蛋白(S. sanguinis),研究了牙龈卟啉单胞菌(P. gingivalis)和核梭状芽胞杆菌(F. nucleatum)。通过时间杀灭测定法检测了对浮游细菌的杀灭活性。通过活/死染色,菌落形成单位(CFU)和代谢活性测试牙本质上的单种4天生物膜。结果六边形的UCNPs @ TiO2的平均直径为39.7nm。UCNPs @ TiO2纳米颗粒表面带正电(+ 12.4mV),具有生物相容性且无细胞毒性。在近红外光(980nm)的激发下,NaYF4:Yb3 +,Tm3 + UCNPs核心可以发出强烈的紫外线(UV),通过能量转移进一步触发了壳TiO2的aPDT功能,从而实现了对浮游生物和细菌的显着抗菌作用。牙周炎相关病原体的生物膜。NIR触发的UCNPs @ TiO2的生物膜减少比对照大得多(p <0.05)。通过近红外触发的aPDT,生物膜CFU降低了3-4个数量级,这明显大于阴性对照组和商业aPDT对照组的生物膜CFU。基于UCNPs @ TiO2的aPDT对这三个物种的杀伤力排名为:S. sanguinis
更新日期:2019-09-22
中文翻译:

新型纳米技术和近红外光动力疗法可杀死与牙周炎相关的生物膜病原体并保护牙周病。
目的随着人口的老龄化,牙周组织的破坏和牙齿脱落越来越成为一个世界性的问题。牙周炎是由细菌感染和生物膜斑块堆积引起的。因此,本研究的目的是:(1)开发近红外(NIR)触发的上转换纳米颗粒和TiO2(UCNPs @ TiO2)的核-壳纳米结构,以及(2)通过抗菌光动力学研究其抑制作用牙周炎相关病原体的治疗(aPDT)。方法通过热分解合成β-NaYF4:Yb3 +,Tm3 +核,并通过水热法用TiO2壳进一步修饰。研究了980nm激光的核-壳结构和上转换荧光诱导的aPDT处理。三种与牙周炎相关的病原体链球菌血红蛋白(S. sanguinis),研究了牙龈卟啉单胞菌(P. gingivalis)和核梭状芽胞杆菌(F. nucleatum)。通过时间杀灭测定法检测了对浮游细菌的杀灭活性。通过活/死染色,菌落形成单位(CFU)和代谢活性测试牙本质上的单种4天生物膜。结果六边形的UCNPs @ TiO2的平均直径为39.7nm。UCNPs @ TiO2纳米颗粒表面带正电(+ 12.4mV),具有生物相容性且无细胞毒性。在近红外光(980nm)的激发下,NaYF4:Yb3 +,Tm3 + UCNPs核心可以发出强烈的紫外线(UV),通过能量转移进一步触发了壳TiO2的aPDT功能,从而实现了对浮游生物和细菌的显着抗菌作用。牙周炎相关病原体的生物膜。NIR触发的UCNPs @ TiO2的生物膜减少比对照大得多(p <0.05)。通过近红外触发的aPDT,生物膜CFU降低了3-4个数量级,这明显大于阴性对照组和商业aPDT对照组的生物膜CFU。基于UCNPs @ TiO2的aPDT对这三个物种的杀伤力排名为:S. sanguinis









































 京公网安备 11010802027423号
京公网安备 11010802027423号