当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of a bifunctional Zn(ii)–organic framework containing a basic amine functionality for selective capture and room temperature fixation of CO2†
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2019-09-20 , DOI: 10.1039/c9qi01058k Rajesh Das 1, 2, 3, 4 , Sandeep Singh Dhankhar 1, 2, 3, 4 , C. M. Nagaraja 1, 2, 3, 4
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2019-09-20 , DOI: 10.1039/c9qi01058k Rajesh Das 1, 2, 3, 4 , Sandeep Singh Dhankhar 1, 2, 3, 4 , C. M. Nagaraja 1, 2, 3, 4
Affiliation
|
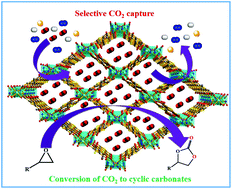
Selective carbon capture and utilization (CCU) as a C1 feedstock for the synthesis of value-added chemicals under ambient conditions catalyzed by porous MOFs constitutes one of the most promising solutions to mitigate the growing CO2 concentration in the atmosphere. Consequently, the synthesis of a novel 3D, microporous, bifunctional Zn(II)–organic framework, {[Zn2(TDC)2(DATRZ)]·(3H2O)·(DMF)}n (Zn-DAT) (where TDC = 2,5-thiophene dicarboxylate ion and DATRZ = 3,5-diamino-1,2,4-triazole), was achieved using a mixed ligand strategy. Single crystal X-ray structural analysis of the MOF revealed the presence of a 3D microporous structure with two types of 1D channels of dimensions 12.5 × 8.7 Å2 and 7.0 × 4.8 Å2 along the crystallographic c- and b-axes, respectively. The presence of basic –NH2 functionalized pores in Zn-DAT induces a selective adsorption property of CO2 with a high heat of adsorption (Qst) value of 39.5 kJ mol−1 which is supported by a theoretically computed binding energy (BE) of 40.9 kJ mol−1. Interestingly, the Qst value observed for Zn-DAT is about 8 kJ mol−1 higher than that of the analogous MOF {[Zn2(TDC)(TRZ)2]·(DMA)·(MeOH)}n (Zn-TAZ) containing the 1,2,4-triazole (TAZ) linker, which highlights the critical role of –NH2 groups in enhancing the interaction energy for CO2. The significantly high value of Qst can be attributed to the stronger interaction of the acidic CO2 molecule with the basic –NH2 groups present in the 1D channels of the Zn-DAT MOF. Furthermore, the presence of both Lewis acidic Zn2+ ions and basic –NH2 groups resulted in the Zn-DAT MOF as an efficient heterogeneous catalyst for chemical fixation of CO2 into cyclic carbonates under mild conditions at RT. Herein, we report a rare example of porous MOFs for the capture and utilization of CO2 at RT and the influence of basic –NH2 groups on the high value of Qst and catalytic conversion of carbon dioxide.
中文翻译:

双官能的Zn(施工II包含用于选择性捕获和CO的室温下固定碱性胺官能团) -有机构架2 †
选择性碳捕获和利用(CCU)作为在多孔MOF催化下在环境条件下合成增值化学品的C1原料,是缓解大气中不断增长的CO 2浓度的最有希望的解决方案之一。因此,合成了一种新型的3D,微孔,双功能Zn(II)-有机骨架,{[Zn 2(TDC)2(DATRZ)]·(3H 2 O)·(DMF)} n(Zn-DAT)(其中TDC = 2,5-噻吩二羧酸根离子,而DATRZ = 3,5-二氨基-1,2,4-三唑)是使用混合配体策略实现的。在MOF的单晶X-射线结构分析显示一个三维微孔结构的存在下用两种类型的尺寸12.5×8.7埃的1D通道2和7.0×4.8埃2沿晶体Ç -和b分别-axes。Zn-DAT中碱性-NH 2官能化孔的存在诱导了具有29.5 kJ mol -1的高吸附热(Q st)值的CO 2的选择性吸附特性。通过理论计算的40.9 kJ mol -1的结合能(BE)来支持。有趣的是,Q ST为观测值的Zn-DAT为约8千焦摩尔-1比类似的MOF的较高{[锌2(TDC)(TRZ)2 ]·(DMA)·(甲醇)} Ñ(含有Zn TAZ)含有1,2,4-三唑(TAZ)接头,其中突出-NH中的关键作用2基团在增强的相互作用能量的CO 2。Q st的极高值可归因于酸性CO 2的较强相互作用Zn-DAT MOF的1D通道中存在带有碱性–NH 2基团的分子。此外,路易斯酸性Zn 2+离子和碱性-NH 2基团的存在导致Zn-DAT MOF作为在室温下温和条件下将CO 2化学固定为环状碳酸酯的有效多相催化剂。在这里,我们报告了一个罕见的多孔MOF实例,用于在RT下捕获和利用CO 2以及碱性-NH 2基团对Q st的高值和二氧化碳的催化转化的影响。
更新日期:2020-01-14
中文翻译:

双官能的Zn(施工II包含用于选择性捕获和CO的室温下固定碱性胺官能团) -有机构架2 †
选择性碳捕获和利用(CCU)作为在多孔MOF催化下在环境条件下合成增值化学品的C1原料,是缓解大气中不断增长的CO 2浓度的最有希望的解决方案之一。因此,合成了一种新型的3D,微孔,双功能Zn(II)-有机骨架,{[Zn 2(TDC)2(DATRZ)]·(3H 2 O)·(DMF)} n(Zn-DAT)(其中TDC = 2,5-噻吩二羧酸根离子,而DATRZ = 3,5-二氨基-1,2,4-三唑)是使用混合配体策略实现的。在MOF的单晶X-射线结构分析显示一个三维微孔结构的存在下用两种类型的尺寸12.5×8.7埃的1D通道2和7.0×4.8埃2沿晶体Ç -和b分别-axes。Zn-DAT中碱性-NH 2官能化孔的存在诱导了具有29.5 kJ mol -1的高吸附热(Q st)值的CO 2的选择性吸附特性。通过理论计算的40.9 kJ mol -1的结合能(BE)来支持。有趣的是,Q ST为观测值的Zn-DAT为约8千焦摩尔-1比类似的MOF的较高{[锌2(TDC)(TRZ)2 ]·(DMA)·(甲醇)} Ñ(含有Zn TAZ)含有1,2,4-三唑(TAZ)接头,其中突出-NH中的关键作用2基团在增强的相互作用能量的CO 2。Q st的极高值可归因于酸性CO 2的较强相互作用Zn-DAT MOF的1D通道中存在带有碱性–NH 2基团的分子。此外,路易斯酸性Zn 2+离子和碱性-NH 2基团的存在导致Zn-DAT MOF作为在室温下温和条件下将CO 2化学固定为环状碳酸酯的有效多相催化剂。在这里,我们报告了一个罕见的多孔MOF实例,用于在RT下捕获和利用CO 2以及碱性-NH 2基团对Q st的高值和二氧化碳的催化转化的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号