Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes
JAMA ( IF 63.1 ) Pub Date : 2019-09-24 , DOI: 10.1001/jama.2019.13772 Julio Rosenstock 1, 2 , Steven E Kahn 3, 4 , Odd Erik Johansen 5 , Bernard Zinman 6, 7 , Mark A Espeland 8 , Hans J Woerle 9 , Egon Pfarr 10 , Annett Keller 10 , Michaela Mattheus 10 , David Baanstra 11 , Thomas Meinicke 12 , Jyothis T George 10 , Maximilian von Eynatten 10 , Darren K McGuire 2 , Nikolaus Marx 13 ,
JAMA ( IF 63.1 ) Pub Date : 2019-09-24 , DOI: 10.1001/jama.2019.13772 Julio Rosenstock 1, 2 , Steven E Kahn 3, 4 , Odd Erik Johansen 5 , Bernard Zinman 6, 7 , Mark A Espeland 8 , Hans J Woerle 9 , Egon Pfarr 10 , Annett Keller 10 , Michaela Mattheus 10 , David Baanstra 11 , Thomas Meinicke 12 , Jyothis T George 10 , Maximilian von Eynatten 10 , Darren K McGuire 2 , Nikolaus Marx 13 ,
Affiliation
|
Importance
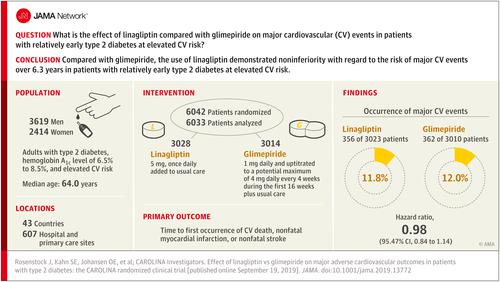
Type 2 diabetes is associated with increased cardiovascular risk. In placebo-controlled cardiovascular safety trials, the dipeptidyl peptidase-4 inhibitor linagliptin demonstrated noninferiority, but it has not been tested against an active comparator. Objective
This trial assessed cardiovascular outcomes of linagliptin vs glimepiride (sulfonylurea) in patients with relatively early type 2 diabetes and risk factors for or established atherosclerotic cardiovascular disease. Design, Setting, and Participants
Randomized, double-blind, active-controlled, noninferiority trial, with participant screening from November 2010 to December 2012, conducted at 607 hospital and primary care sites in 43 countries involving 6042 participants. Adults with type 2 diabetes, glycated hemoglobin of 6.5% to 8.5%, and elevated cardiovascular risk were eligible for inclusion. Elevated cardiovascular risk was defined as documented atherosclerotic cardiovascular disease, multiple cardiovascular risk factors, aged at least 70 years, and evidence of microvascular complications. Follow-up ended in August 2018. Interventions
Patients were randomized to receive 5 mg of linagliptin once daily (n = 3023) or 1 to 4 mg of glimepiride once daily (n = 3010) in addition to usual care. Investigators were encouraged to intensify glycemic treatment, primarily by adding or adjusting metformin, α-glucosidase inhibitors, thiazolidinediones, or insulin, according to clinical need. Main Outcomes and Measures
The primary outcome was time to first occurrence of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke with the aim to establish noninferiority of linagliptin vs glimepiride, defined by the upper limit of the 2-sided 95.47% CI for the hazard ratio (HR) of linagliptin relative to glimepiride of less than 1.3. Results
Of 6042 participants randomized, 6033 (mean age, 64.0 years; 2414 [39.9%] women; mean glycated hemoglobin, 7.2%; median duration of diabetes, 6.3 years; 42% with macrovascular disease; 59% had undergone metformin monotherapy) were treated and analyzed. The median duration of follow-up was 6.3 years. The primary outcome occurred in 356 of 3023 participants (11.8%) in the linagliptin group and 362 of 3010 (12.0%) in the glimepiride group (HR, 0.98 [95.47% CI, 0.84-1.14]; P < .001 for noninferiority), meeting the noninferiority criterion but not superiority (P = .76). Adverse events occurred in 2822 participants (93.4%) in the linagliptin group and 2856 (94.9%) in the glimepiride group, with 15 participants (0.5%) in the linagliptin group vs 16 (0.5%) in the glimepiride group with adjudicated-confirmed acute pancreatitis. At least 1 episode of hypoglycemic adverse events occurred in 320 (10.6%) participants in the linagliptin group and 1132 (37.7%) in the glimepiride group (HR, 0.23 [95% CI, 0.21-0.26]). Conclusions and Relevance
Among adults with relatively early type 2 diabetes and elevated cardiovascular risk, the use of linagliptin compared with glimepiride over a median 6.3 years resulted in a noninferior risk of a composite cardiovascular outcome. Trial Registration
ClinicalTrials.gov Identifier: NCT01243424.
中文翻译:

利格列汀与格列美脲对 2 型糖尿病患者主要不良心血管结局的影响
重要性 2 型糖尿病与心血管风险增加相关。在安慰剂对照的心血管安全性试验中,二肽基肽酶 4 抑制剂利格列汀表现出非劣效性,但尚未针对活性比较药物进行测试。目的 本试验评估了利格列汀与格列美脲(磺酰脲类)治疗相对早期 2 型糖尿病以及动脉粥样硬化性心血管疾病危险因素的患者的心血管结局。设计、设置和参与者 2010 年 11 月至 2012 年 12 月期间,在 43 个国家的 607 家医院和初级保健机构进行了随机、双盲、主动对照、非劣效性试验,参与者筛选,涉及 6042 名参与者。患有 2 型糖尿病、糖化血红蛋白为 6.5% 至 8.5% 且心血管风险较高的成人有资格入选。心血管风险升高的定义是有记录的动脉粥样硬化性心血管疾病、多种心血管危险因素、年龄至少 70 岁以及微血管并发症的证据。随访于 2018 年 8 月结束。 干预措施 除常规护理外,患者被随机分为每日一次 5 毫克利格列汀 (n = 3023) 或每日一次 1 至 4 毫克格列美脲 (n = 3010)。鼓励研究人员加强血糖治疗,主要是根据临床需要添加或调整二甲双胍、α-葡萄糖苷酶抑制剂、噻唑烷二酮类或胰岛素。主要结果和措施 主要结果是首次发生心血管死亡、非致命性心肌梗死或非致命性卒中的时间,目的是确定利格列汀与格列美脲的非劣效性,定义为危险的双侧 95.47% CI 上限利格列汀相对于格列美脲的比率(HR)小于1.3。结果 6042 名随机参与者中,有 6033 名(平均年龄 64.0 岁;2414 名女性 [39.9%];平均糖化血红蛋白 7.2%;糖尿病中位病程 6.3 年;42% 患有大血管疾病;59% 接受过二甲双胍单药治疗)进行处理和分析。中位随访时间为 6.3 年。主要结局发生在利格列汀组 3023 名参与者中的 356 名 (11.8%) 和格列美脲组 3010 名参与者中的 362 名 (12.0%)(HR,0.98 [95.47% CI,0.84-1.14];非劣效性 P < .001) ,满足非劣效性标准,但不满足优效性 (P = .76)。利格列汀组有 2822 名参与者(93.4%)发生不良事件,格列美脲组有 2856 名参与者(94.9%)发生不良事件,其中利格列汀组有 15 名参与者(0.5%),格列美脲组有 16 名参与者(0.5%),经裁定确认急性胰腺炎。利格列汀组 320 名 (10.6%) 参与者和格列美脲组 1132 名 (37.7%) 参与者至少发生 1 次低血糖不良事件(HR,0.23 [95% CI,0.21-0.26])。结论和相关性 在患有相对早期 2 型糖尿病且心血管风险较高的成年人中,与格列美脲相比,利格列汀的使用中位时间为 6.3 年,导致复合心血管结局的风险不较低。试验注册 ClinicalTrials.gov 标识符:NCT01243424。
更新日期:2019-09-24
中文翻译:

利格列汀与格列美脲对 2 型糖尿病患者主要不良心血管结局的影响
重要性 2 型糖尿病与心血管风险增加相关。在安慰剂对照的心血管安全性试验中,二肽基肽酶 4 抑制剂利格列汀表现出非劣效性,但尚未针对活性比较药物进行测试。目的 本试验评估了利格列汀与格列美脲(磺酰脲类)治疗相对早期 2 型糖尿病以及动脉粥样硬化性心血管疾病危险因素的患者的心血管结局。设计、设置和参与者 2010 年 11 月至 2012 年 12 月期间,在 43 个国家的 607 家医院和初级保健机构进行了随机、双盲、主动对照、非劣效性试验,参与者筛选,涉及 6042 名参与者。患有 2 型糖尿病、糖化血红蛋白为 6.5% 至 8.5% 且心血管风险较高的成人有资格入选。心血管风险升高的定义是有记录的动脉粥样硬化性心血管疾病、多种心血管危险因素、年龄至少 70 岁以及微血管并发症的证据。随访于 2018 年 8 月结束。 干预措施 除常规护理外,患者被随机分为每日一次 5 毫克利格列汀 (n = 3023) 或每日一次 1 至 4 毫克格列美脲 (n = 3010)。鼓励研究人员加强血糖治疗,主要是根据临床需要添加或调整二甲双胍、α-葡萄糖苷酶抑制剂、噻唑烷二酮类或胰岛素。主要结果和措施 主要结果是首次发生心血管死亡、非致命性心肌梗死或非致命性卒中的时间,目的是确定利格列汀与格列美脲的非劣效性,定义为危险的双侧 95.47% CI 上限利格列汀相对于格列美脲的比率(HR)小于1.3。结果 6042 名随机参与者中,有 6033 名(平均年龄 64.0 岁;2414 名女性 [39.9%];平均糖化血红蛋白 7.2%;糖尿病中位病程 6.3 年;42% 患有大血管疾病;59% 接受过二甲双胍单药治疗)进行处理和分析。中位随访时间为 6.3 年。主要结局发生在利格列汀组 3023 名参与者中的 356 名 (11.8%) 和格列美脲组 3010 名参与者中的 362 名 (12.0%)(HR,0.98 [95.47% CI,0.84-1.14];非劣效性 P < .001) ,满足非劣效性标准,但不满足优效性 (P = .76)。利格列汀组有 2822 名参与者(93.4%)发生不良事件,格列美脲组有 2856 名参与者(94.9%)发生不良事件,其中利格列汀组有 15 名参与者(0.5%),格列美脲组有 16 名参与者(0.5%),经裁定确认急性胰腺炎。利格列汀组 320 名 (10.6%) 参与者和格列美脲组 1132 名 (37.7%) 参与者至少发生 1 次低血糖不良事件(HR,0.23 [95% CI,0.21-0.26])。结论和相关性 在患有相对早期 2 型糖尿病且心血管风险较高的成年人中,与格列美脲相比,利格列汀的使用中位时间为 6.3 年,导致复合心血管结局的风险不较低。试验注册 ClinicalTrials.gov 标识符:NCT01243424。









































 京公网安备 11010802027423号
京公网安备 11010802027423号