当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The effects of active site and support on hydrogen elimination over transition-metal-functionalized yttria-decorated metal–organic frameworks
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2019-09-19 , DOI: 10.1039/c9cy01069f Bo Yang 1, 2, 3, 4, 5 , Kamal Sharkas 1, 2, 3, 4, 5 , Laura Gagliardi 1, 2, 3, 4, 5 , Donald G. Truhlar 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2019-09-19 , DOI: 10.1039/c9cy01069f Bo Yang 1, 2, 3, 4, 5 , Kamal Sharkas 1, 2, 3, 4, 5 , Laura Gagliardi 1, 2, 3, 4, 5 , Donald G. Truhlar 1, 2, 3, 4, 5
Affiliation
|
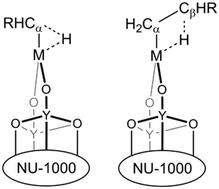
Hydrogen elimination from a metal alkyl complex and its reverse reaction are critical elementary steps in many catalytic cycles. In understanding such catalytic cycles, it is important to learn about the effect of the active site as well as effects beyond the active site, especially support effects. Here we employ density-functional-theory-based computational screening of transition-metal-functionalized yttria-decorated NU-1000 metal–organic frameworks to study the effect of metal sites and supports on the hydrogen elimination reactions. We consider six transition metals. The screening shows that a Nb-based catalyst has the lowest free energy of activation for both α-H elimination and β-H elimination, but for the latter we must also consider the free energy of release of the alkene. By the Sabatier principle, the optimal catalyst for β-H elimination is the one that interacts with molecules with intermediate strength for easy reactant activation as well as easy product desorption. By employing a volcano plot to find the optimum compromise, we identified V-functionalized, yttria-decorated NU-1000 as the most active catalyst among the trial candidates for the β-H elimination reaction. The analysis also reveals a support-specific charge transfer process in which the yttria and carboxylate linkers enable electron transfer between the transition-metal site and organic linkers of the NU-1000 simultaneously with a coordinated change of spin at the metal site and in the linker groups. Based on our analyses, we hypothesize that the observed charge transfer enables certain functional groups, such as propyl, to interact more strongly with the support and stabilize the catalyst.
中文翻译:

过渡金属官能化的氧化钇修饰的金属有机框架上活性位点和支持物对氢消除的影响
在许多催化循环中,从烷基金属配合物中除去氢及其逆反应是关键的基本步骤。在理解这种催化循环时,重要的是要了解活性位点的作用以及活性位点以外的作用,特别是支持作用。在这里,我们采用基于密度泛函理论的过渡金属官能化氧化钇修饰的NU-1000金属有机框架的计算筛选,研究了金属位点和载体对氢消除反应的影响。我们考虑了六种过渡金属。筛选显示,基于Nb的催化剂对于α-H消除和β-H消除均具有最低的活化自由能,但对于后者,我们还必须考虑烯烃释放的自由能。根据Sabatier原则,消除β-H的最佳催化剂是与具有中等强度的分子相互作用的催化剂,可轻松激活反应物并易于产物解吸。通过使用火山图找到最佳折衷方案,我们确定了V-官能化,氧化钇修饰的NU-1000是β-H消除反应的试验候选物中活性最高的催化剂。该分析还揭示了一种特定于支持物的电荷转移过程,其中氧化钇和羧酸盐连接子使NU-1000的过渡金属位点与有机连接子之间能够进行电子转移,同时在金属位点和连接子中自旋的协调变化组。根据我们的分析,我们假设观察到的电荷转移可以使某些官能团(例如丙基,
更新日期:2019-09-19
中文翻译:

过渡金属官能化的氧化钇修饰的金属有机框架上活性位点和支持物对氢消除的影响
在许多催化循环中,从烷基金属配合物中除去氢及其逆反应是关键的基本步骤。在理解这种催化循环时,重要的是要了解活性位点的作用以及活性位点以外的作用,特别是支持作用。在这里,我们采用基于密度泛函理论的过渡金属官能化氧化钇修饰的NU-1000金属有机框架的计算筛选,研究了金属位点和载体对氢消除反应的影响。我们考虑了六种过渡金属。筛选显示,基于Nb的催化剂对于α-H消除和β-H消除均具有最低的活化自由能,但对于后者,我们还必须考虑烯烃释放的自由能。根据Sabatier原则,消除β-H的最佳催化剂是与具有中等强度的分子相互作用的催化剂,可轻松激活反应物并易于产物解吸。通过使用火山图找到最佳折衷方案,我们确定了V-官能化,氧化钇修饰的NU-1000是β-H消除反应的试验候选物中活性最高的催化剂。该分析还揭示了一种特定于支持物的电荷转移过程,其中氧化钇和羧酸盐连接子使NU-1000的过渡金属位点与有机连接子之间能够进行电子转移,同时在金属位点和连接子中自旋的协调变化组。根据我们的分析,我们假设观察到的电荷转移可以使某些官能团(例如丙基,











































 京公网安备 11010802027423号
京公网安备 11010802027423号