Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial.
The Lancet ( IF 98.4 ) Pub Date : 2019-09-18 , DOI: 10.1016/s0140-6736(19)32131-2 David R Matthews 1 , Päivi M Paldánius 2 , Pieter Proot 2 , YannTong Chiang 3 , Michael Stumvoll 4 , Stefano Del Prato 5 ,
The Lancet ( IF 98.4 ) Pub Date : 2019-09-18 , DOI: 10.1016/s0140-6736(19)32131-2 David R Matthews 1 , Päivi M Paldánius 2 , Pieter Proot 2 , YannTong Chiang 3 , Michael Stumvoll 4 , Stefano Del Prato 5 ,
Affiliation
|
BACKGROUND
Early treatment intensification leading to sustained good glycaemic control is essential to delay diabetic complications. Although initial combination therapy has been suggested to offer more opportunities than a traditional stepwise approach, its validity remains to be determined.
METHODS
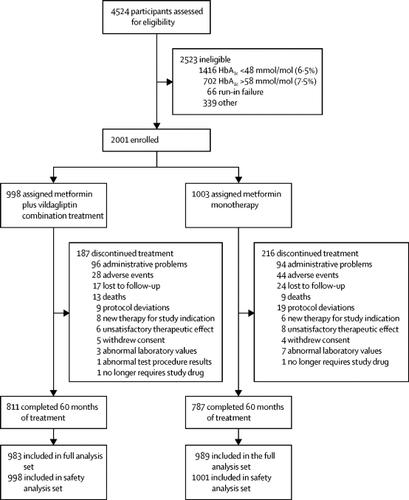
Vildagliptin Efficacy in combination with metfoRmIn For earlY treatment of type 2 diabetes (VERIFY) was a randomised, double-blind, parallel-group study of newly diagnosed patients with type 2 diabetes conducted in 254 centres across 34 countries. The study consisted of a 2-week screening visit, a 3-week metformin-alone run-in period, and a 5-year treatment period, which was further split into study periods 1, 2, and 3. Patients aged 18-70 years were included if they had type 2 diabetes diagnosed within 2 years prior to enrolment, and centrally confirmed glycated haemoglobin A1c (HbA1c) of 48-58 mmol/mol (6·5-7·5%) and a body-mass index of 22-40 kg/m2. Patients were randomly assigned in a 1:1 ratio either to the early combination treatment group or to the initial metformin monotherapy group, with the help of an interactive response technology system and simple randomisation without stratification. Patients, investigators, clinical staff performing the assessments, and data analysts were masked to treatment allocation. In study period 1, patients received either the early combination treatment with metformin (stable daily dose of 1000 mg, 1500 mg, or 2000 mg) and vildagliptin 50 mg twice daily, or standard-of-care initial metformin monotherapy (stable daily dose of 1000 mg, 1500 mg, or 2000 mg) and placebo twice daily. If the initial treatment did not maintain HbA1c below 53 mmol/mol (7·0%), confirmed at two consecutive scheduled visits which were 13 weeks apart, patients in the metformin monotherapy group received vildagliptin 50 mg twice daily in place of the placebo and entered study period 2, during which all patients received the combination therapy. The primary efficacy endpoint was the time from randomisation to initial treatment failure, defined as HbA1c measurement of at least 53 mmol/mol (7·0%) at two consecutive scheduled visits, 13 weeks apart from randomisation through period 1. The full analysis set included patients who received at least one randomised study medication and had at least one post-randomisation efficacy parameter assessed. The safety analysis set included all patients who received at least one dose of randomised study medication. This study is registered with ClinicalTrials.gov, NCT01528254.
FINDINGS
Trial enrolment began on March 30, 2012, and was completed on April 10, 2014. Of the 4524 participants screened, 2001 eligible participants were randomly assigned to either the early combination treatment group (n=998) or the initial metformin monotherapy group (n=1003). A total of 1598 (79·9%) patients completed the 5-year study: 811 (81·3%) in the early combination therapy group and 787 (78·5%) in the monotherapy group. The incidence of initial treatment failure during period 1 was 429 (43·6%) patients in the combination treatment group and 614 (62·1%) patients in the monotherapy group. The median observed time to treatment failure in the monotherapy group was 36·1 (IQR 15·3-not reached [NR]) months, while the median time to treatment failure time for those receiving early combination therapy could only be estimated to be beyond the study duration at 61·9 (29·9-NR) months. A significant reduction in the relative risk for time to initial treatment failure was observed in the early combination treatment group compared with the monotherapy group over the 5-year study duration (hazard ratio 0·51 [95% CI 0·45-0·58]; p<0·0001). Both treatment approaches were safe and well tolerated, with no unexpected or new safety findings, and no deaths related to study treatment.
INTERPRETATION
Early intervention with a combination therapy of vildagliptin plus metformin provides greater and durable long-term benefits compared with the current standard-of-care initial metformin monotherapy for patients with newly diagnosed type 2 diabetes.
FUNDING
Novartis.
中文翻译:

在新诊断的2型糖尿病(VERIFY)中,采用维达列汀和二甲双胍早期联合治疗与序贯二甲双胍单一治疗的血糖耐受性:一项为期5年,多中心,随机,双盲试验。
背景技术导致持续良好的血糖控制的早期治疗强化对于延迟糖尿病并发症是必不可少的。尽管已建议初始联合疗法比传统的逐步治疗方法提供更多的机会,但其有效性仍有待确定。方法维格列汀与二甲双胍联用的疗效早期治疗2型糖尿病(VERIFY)是在34个国家/地区的254个中心进行的一项随机,双盲,平行组研究,对新诊断的2型糖尿病患者进行了研究。该研究包括2周的筛查访视,3周的二甲双胍单独磨合期和5年的治疗期,该治疗期又分为研究期1、2和3。年龄在18-70岁的患者如果他们在入学前2年内被诊断出患有2型糖尿病,则将这些年包括在内,集中确认的糖化血红蛋白A1c(HbA1c)为48-58 mmol / mol(6·5-7·5%),体质量指数为22-40 kg / m2。借助互动反应技术系统和简单的无分层随机分组,将患者以1:1的比例随机分配到早期联合治疗组或初始二甲双胍单药治疗组。患者,研究人员,进行评估的临床人员以及数据分析人员都无法进行治疗分配。在研究阶段1中,患者接受了早期二甲双胍(每日稳定剂量1000 mg,1500 mg或2000 mg)和维达列汀50 mg每日两次的联合治疗,或采用标准的初始二甲双胍单药治疗(每日稳定剂量1000毫克,1500毫克或2000毫克)和安慰剂,每天两次。如果最初的治疗未将HbA1c维持在低于53 mmol / mol(7·0%),在相距13周的两次连续预定访视中得到确认,则二甲双胍单药治疗组的患者每天两次接受维格列汀50 mg代替安慰剂和安慰剂。进入研究期2,在此期间所有患者均接受联合治疗。主要功效终点是从随机分组到初始治疗失败的时间,定义为连续两次定期就诊时的HbA1c测量值至少为53 mmol / mol(7·0%),从第1阶段随机分组到间隔13周。包括接受至少一种随机研究药物并且至少评估了一种随机后功效参数的患者。安全性分析集包括接受至少一剂随机研究药物的所有患者。该研究已在ClinicalTrials.gov注册,NCT01528254。结果自2012年3月30日开始试验,并于2014年4月10日结束。在筛选的4524名参与者中,有2001名合格参与者被随机分配到早期联合治疗组(n = 998)或初始二甲双胍单药治疗组(n = 998)。 n = 1003)。共有1598(79·9%)位患者完成了5年研究:早期联合治疗组为811(81·3%),单药治疗组为787(78·5%)。在第1阶段,联合治疗组的初次治疗失败率为429(43·6%),单药治疗组为614(62·1%)。单药治疗组观察到治疗失败的中位时间为36·1(IQR 15·3-未达到[NR])个月,而接受早期联合治疗的患者中到治疗失败时间的中位数只能估计超过61·9(29·9-NR)个月的研究持续时间。在5年的研究期间,与单一疗法组相比,早期联合治疗组的初始治疗失败时间的相对风险显着降低(危险比0·51 [95%CI 0·45-0·58 ]; p <0·0001)。两种治疗方法都是安全且耐受性良好的,没有意外或新的安全性发现,也没有与研究治疗相关的死亡。解释与新诊断的2型糖尿病患者相比,采用维达列汀加二甲双胍联合治疗的早期干预与当前的标准医疗服务初始二甲双胍单药治疗相比,具有更大,更持久的长期益处。资助诺华。
更新日期:2019-10-25
中文翻译:

在新诊断的2型糖尿病(VERIFY)中,采用维达列汀和二甲双胍早期联合治疗与序贯二甲双胍单一治疗的血糖耐受性:一项为期5年,多中心,随机,双盲试验。
背景技术导致持续良好的血糖控制的早期治疗强化对于延迟糖尿病并发症是必不可少的。尽管已建议初始联合疗法比传统的逐步治疗方法提供更多的机会,但其有效性仍有待确定。方法维格列汀与二甲双胍联用的疗效早期治疗2型糖尿病(VERIFY)是在34个国家/地区的254个中心进行的一项随机,双盲,平行组研究,对新诊断的2型糖尿病患者进行了研究。该研究包括2周的筛查访视,3周的二甲双胍单独磨合期和5年的治疗期,该治疗期又分为研究期1、2和3。年龄在18-70岁的患者如果他们在入学前2年内被诊断出患有2型糖尿病,则将这些年包括在内,集中确认的糖化血红蛋白A1c(HbA1c)为48-58 mmol / mol(6·5-7·5%),体质量指数为22-40 kg / m2。借助互动反应技术系统和简单的无分层随机分组,将患者以1:1的比例随机分配到早期联合治疗组或初始二甲双胍单药治疗组。患者,研究人员,进行评估的临床人员以及数据分析人员都无法进行治疗分配。在研究阶段1中,患者接受了早期二甲双胍(每日稳定剂量1000 mg,1500 mg或2000 mg)和维达列汀50 mg每日两次的联合治疗,或采用标准的初始二甲双胍单药治疗(每日稳定剂量1000毫克,1500毫克或2000毫克)和安慰剂,每天两次。如果最初的治疗未将HbA1c维持在低于53 mmol / mol(7·0%),在相距13周的两次连续预定访视中得到确认,则二甲双胍单药治疗组的患者每天两次接受维格列汀50 mg代替安慰剂和安慰剂。进入研究期2,在此期间所有患者均接受联合治疗。主要功效终点是从随机分组到初始治疗失败的时间,定义为连续两次定期就诊时的HbA1c测量值至少为53 mmol / mol(7·0%),从第1阶段随机分组到间隔13周。包括接受至少一种随机研究药物并且至少评估了一种随机后功效参数的患者。安全性分析集包括接受至少一剂随机研究药物的所有患者。该研究已在ClinicalTrials.gov注册,NCT01528254。结果自2012年3月30日开始试验,并于2014年4月10日结束。在筛选的4524名参与者中,有2001名合格参与者被随机分配到早期联合治疗组(n = 998)或初始二甲双胍单药治疗组(n = 998)。 n = 1003)。共有1598(79·9%)位患者完成了5年研究:早期联合治疗组为811(81·3%),单药治疗组为787(78·5%)。在第1阶段,联合治疗组的初次治疗失败率为429(43·6%),单药治疗组为614(62·1%)。单药治疗组观察到治疗失败的中位时间为36·1(IQR 15·3-未达到[NR])个月,而接受早期联合治疗的患者中到治疗失败时间的中位数只能估计超过61·9(29·9-NR)个月的研究持续时间。在5年的研究期间,与单一疗法组相比,早期联合治疗组的初始治疗失败时间的相对风险显着降低(危险比0·51 [95%CI 0·45-0·58 ]; p <0·0001)。两种治疗方法都是安全且耐受性良好的,没有意外或新的安全性发现,也没有与研究治疗相关的死亡。解释与新诊断的2型糖尿病患者相比,采用维达列汀加二甲双胍联合治疗的早期干预与当前的标准医疗服务初始二甲双胍单药治疗相比,具有更大,更持久的长期益处。资助诺华。










































 京公网安备 11010802027423号
京公网安备 11010802027423号