Communications Chemistry ( IF 5.9 ) Pub Date : 2019-09-17 , DOI: 10.1038/s42004-019-0205-5 Maciej Majewski , Sergio Ruiz-Carmona , Xavier Barril
|
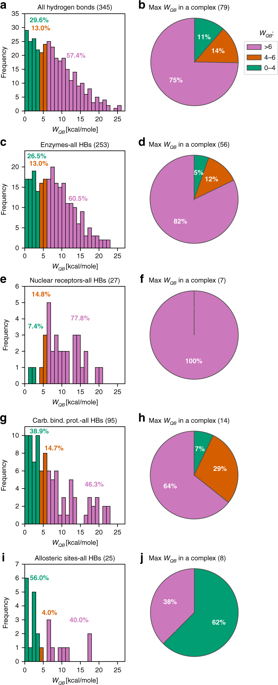
The predominant view in structure-based drug design is that small-molecule ligands, once bound to their target structures, display a well-defined binding mode. However, structural stability (robustness) is not necessary for thermodynamic stability (binding affinity). In fact, it entails an entropic penalty that counters complex formation. Surprisingly, little is known about the causes, consequences and real degree of robustness of protein-ligand complexes. Since hydrogen bonds have been described as essential for structural stability, here we investigate 469 such interactions across two diverse structure sets, comprising of 79 drug-like and 27 fragment ligands, respectively. Completely constricted protein-ligand complexes are rare and may fulfill a functional role. Most complexes balance order and disorder by combining a single anchoring point with looser regions. 25% do not contain any robust hydrogen bond and may form loose structures. Structural stability analysis reveals a hidden layer of complexity in protein-ligand complexes that should be considered in ligand design.
中文翻译:

对蛋白质-配体复合物的结构稳定性的研究揭示了有序和无序之间的平衡
基于结构的药物设计的主要观点是,小分子配体一旦结合到其靶结构,就会显示出明确定义的结合模式。但是,结构稳定性(鲁棒性)对于热力学稳定性(结合亲和力)不是必需的。实际上,它带来了对抗复杂形成的熵罚。令人惊讶的是,关于蛋白质-配体复合物的原因,结果和坚固性的真实程度知之甚少。由于氢键已被描述为结构稳定性必不可少的,在这里我们研究了跨越两个不同结构集的469种此类相互作用,分别包括79种药物样配体和27个片段配体。完全收缩的蛋白质-配体复合物很少,并且可能发挥功能性作用。大多数复合体通过将单个锚固点与较宽松的区域相结合来平衡秩序和无序状态。25%不包含任何牢固的氢键,并可能形成疏松结构。结构稳定性分析揭示了配体设计中应考虑的蛋白质-配体复合物的复杂性的隐藏层。











































 京公网安备 11010802027423号
京公网安备 11010802027423号