JAMA Neurology ( IF 29.0 ) Pub Date : 2020-01-01 , DOI: 10.1001/jamaneurol.2019.2984 Kanjana S Perera 1 , Kelvin K H Ng 1 , Sumiti Nayar 1 , Luciana Catanese 1 , Leanne Dyal 1 , Mukul Sharma 1 , Stuart J Connolly 1 , Salim Yusuf 1 , Jackie Bosch 1 , John W Eikelboom 1 , Robert G Hart 1
|
Importance The COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) randomized clinical trial was stopped early owing to the efficacy of low-dose rivaroxaban plus aspirin in preventing major cardiovascular events. The main reason for early trial termination was the effect of combination therapy on reducing ischemic strokes.
Objective To analyze the association between low-dose rivaroxaban with or without aspirin and different ischemic stroke subtypes.
Design, Setting, and Participants This is a secondary analysis of a multicenter, double-blind, randomized, placebo-controlled study that was performed in 33 countries from March 12, 2013, to May 10, 2016. Patients with stable atherosclerotic vascular disease were eligible, and a total of 27 395 participants were randomized and followed up to February 6, 2017. All first ischemic strokes and uncertain strokes that occurred by this date were adjudicated using TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria. The analysis of ischemic stroke subtypes was evaluated using an intention-to-treat principle. Statistical analysis was performed from March 12, 2013, to February 6, 2017.
Interventions Participants received rivaroxaban (2.5 mg twice a day) plus aspirin (100 mg once a day), rivaroxaban (5 mg twice a day), or aspirin (100 mg once a day).
Main Outcomes and Measures Risk of ischemic stroke subtypes during follow-up.
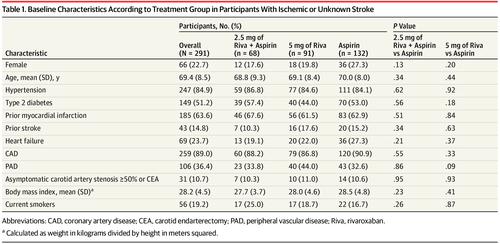
Results A total of 291 patients (66 women; mean [SD] age, 69.4 [8.5] years; 43 [14.8%] had a previous nonlacunar stroke) experienced an ischemic stroke. During the study, 49 patients (16.8%) received a diagnosis of atrial fibrillation. Applying TOAST criteria, 59 strokes (20.3%) were cardioembolic, 54 strokes (18.6%) were secondary to greater than 50% stenosis of the ipsilateral internal carotid artery, 42 strokes (14.4%) had a negative evaluation that met criteria for embolic stroke of undetermined source, and 21 strokes (7.2%) were secondary to small vessel disease. There were significantly fewer cardioembolic strokes (hazard ratio [HR], 0.40 [95% CI, 0.20-0.78]; P = .005) and embolic strokes of undetermined source (HR, 0.30 [95% CI, 0.12-0.74]; P = .006) in the combination therapy group compared with the aspirin-only group. A trend for reduction in strokes secondary to small vessel disease (HR, 0.36 [95% CI, 0.12-1.14]; P = .07) was not statistically significant. No significant difference was observed between the 2 groups in strokes secondary to greater than 50% carotid artery stenosis (HR, 0.85 [95% CI, 0.45-1.60]; P = .61). Rivaroxaban, 5 mg, twice daily showed a trend for reducing cardioembolic strokes compared with aspirin (HR, 0.57 [95% CI, 0.31-1.03]; P = .06) but was not associated with reducing other stroke subtypes.
Conclusions and Relevance For patients with systemic atherosclerosis, low-dose rivaroxaban plus aspirin was associated with large, significant reductions in cardioembolic strokes and embolic strokes of undetermined source. However, these results of exploratory analysis need to be independently confirmed before influencing clinical practice.
Trial Registration ClinicalTrials.gov identifier: NCT01776424
中文翻译:

有或没有阿司匹林的低剂量利伐沙班与缺血性卒中亚型之间的关联:COMPASS试验的二级分析。
重要性 由于低剂量利伐沙班加阿司匹林在预防重大心血管事件中的功效,COMPASS(使用抗凝策略的人的心血管结局)随机临床试验已提前停止。提前终止试验的主要原因是联合治疗对减少缺血性卒中的作用。
目的 分析具有或不具有阿司匹林的小剂量利伐沙班与不同缺血性中风亚型之间的关系。
设计,背景和参与者 这是一项多中心,双盲,随机,安慰剂对照研究的二级分析,该研究于2013年3月12日至2016年5月10日在33个国家进行。共有27395名参与者被随机分组,并随访至2017年2月6日。使用TOAST(急性卒中治疗中的组织10172试验)对截至该日期发生的所有首发缺血性卒中和不确定性卒中进行判定。缺血性中风亚型的分析使用意向性治疗原则进行评估。从2013年3月12日至2017年2月6日进行统计分析。
干预措施 参与者接受利伐沙班(每天两次2.5毫克)加阿司匹林(每天一次100毫克),利伐沙班(每天两次5毫克)或阿司匹林(每天一次100毫克)。
主要结果和措施 随访期间存在缺血性中风亚型的风险。
结果 总共291例患者发生了缺血性中风(66名女性;平均[SD]年龄为69.4 [8.5]岁; 43名[14.8%]患有先前的非腔隙性中风)。在研究过程中,有49位患者(16.8%)被诊断为房颤。应用TOAST标准,心脏栓塞59例(20.3%),同侧颈内动脉大于50%狭窄继发54例(18.6%),符合栓塞性中风标准的阴性评估为42例(14.4%)来源不明,其中21例(7.2%)中风是继发于小血管疾病。心脏栓塞卒中(危险比[HR],0.40 [95%CI,0.20-0.78];P = .005)和未确定来源的栓塞卒中(HR,0.30 [95%CI,0.12-0.74];P = 0.006)与单纯阿司匹林组比较。继发于小血管疾病的卒中减少的趋势(HR,0.36 [95%CI,0.12-1.14];P = .07)在统计学上不显着。在颈动脉狭窄大于50%的继发卒中中,两组之间未观察到显着差异(HR,0.85 [95%CI,0.45-1.60];P = 0.61 )。与阿司匹林相比,每日两次5 mg利伐沙班与阿司匹林相比有降低心脏栓塞性中风的趋势(HR,0.57 [95%CI,0.31-1.03];P = .06),但与减少其他中风亚型无关。
结论和相关性 对于系统性动脉粥样硬化患者,低剂量利伐沙班加阿司匹林可显着减少心脏栓塞性卒中和不确定来源的栓塞性卒中。但是,探索性分析的这些结果需要在影响临床实践之前进行独立确认。
试验注册 ClinicalTrials.gov标识符:NCT01776424



























 京公网安备 11010802027423号
京公网安备 11010802027423号