当前位置:
X-MOL 学术
›
J. Trace Elem. Med. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The role of poly(ADP-ribose) polymerases in manganese exposed Caenorhabditis elegans.
Journal of Trace Elements in Medicine and Biology ( IF 3.6 ) Pub Date : 2019-09-14 , DOI: 10.1016/j.jtemb.2019.09.001 Catherine Neumann 1 , Jessica Baesler 2 , Gereon Steffen 1 , Merle Marie Nicolai 3 , Tabea Zubel 4 , Michael Aschner 5 , Alexander Bürkle 4 , Aswin Mangerich 4 , Tanja Schwerdtle 2 , Julia Bornhorst 6
Journal of Trace Elements in Medicine and Biology ( IF 3.6 ) Pub Date : 2019-09-14 , DOI: 10.1016/j.jtemb.2019.09.001 Catherine Neumann 1 , Jessica Baesler 2 , Gereon Steffen 1 , Merle Marie Nicolai 3 , Tabea Zubel 4 , Michael Aschner 5 , Alexander Bürkle 4 , Aswin Mangerich 4 , Tanja Schwerdtle 2 , Julia Bornhorst 6
Affiliation
|
BACKGROUND AND AIM
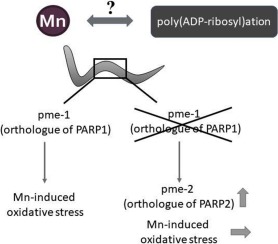
When exceeding the homeostatic range, manganese (Mn) might cause neurotoxicity, characteristic of the pathophysiology of several neurological diseases. Although the underlying mechanism of its neurotoxicity remains unclear, Mn-induced oxidative stress contributes to disease etiology. DNA damage caused by oxidative stress may further trigger dysregulation of DNA-damage-induced poly(ADP-ribosyl)ation (PARylation), which is of central importance especially for neuronal homeostasis. Accordingly, this study was designed to assess in the genetically traceable in vivo model Caenorhabditis elegans the role of PARylation as well as the consequences of loss of pme-1 or pme-2 (orthologues of PARP1 and PARP2) in Mn-induced toxicity.
METHODS
A specific and sensitive isotope-dilution liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was developed to quantify PARylation in worms. Next to monitoring the PAR level, pme-1 and pme-2 gene expression as well as Mn-induced oxidative stress was studied in wildtype worms and the pme deletion mutants.
RESULTS AND CONCLUSION
While Mn failed to induce PARylation in wildtype worms, toxic doses of Mn led to PAR-induction in pme-1-deficient worms, due to an increased gene expression of pme-2 in the pme-1 deletion mutants. However, this effect could not be observed at sub-toxic Mn doses as well as upon longer incubation times. Regarding Mn-induced oxidative stress, the deletion mutants did not show hypersensitivity. Taken together, this study characterizes worms to model PAR inhibition and addresses the consequences for Mn-induced oxidative stress in genetically manipulated worms.
中文翻译:

聚(ADP-核糖)聚合酶在锰暴露的秀丽隐杆线虫中的作用。
背景和目的 当超过稳态范围时,锰 (Mn) 可能会引起神经毒性,这是几种神经系统疾病的病理生理学特征。尽管其神经毒性的潜在机制尚不清楚,但锰诱导的氧化应激有助于疾病病因学。氧化应激引起的 DNA 损伤可能进一步引发 DNA 损伤诱导的聚(ADP-核糖基)化(PARylation)失调,这对于神经元稳态至关重要。因此,本研究旨在评估遗传可追溯的体内模型秀丽隐杆线虫中 PARylation 的作用以及 pme-1 或 pme-2(PARP1 和 PARP2 的直系同源物)丢失在锰诱导的毒性中的后果。方法开发了一种特异且灵敏的同位素稀释液相色谱-串联质谱 (LC-MS/MS) 方法来定量蠕虫中的 PARylation。接下来监测 PAR 水平,在野生型线虫和 pme 缺失突变体中研究了 pme-1 和 pme-2 基因表达以及 Mn 诱导的氧化应激。结果和结论 虽然 Mn 未能在野生型蠕虫中诱导 PARylation,但由于 pme-1 缺失突变体中 pme-2 基因表达增加,有毒剂量的 Mn 导致 pme-1 缺陷型蠕虫中 PAR 诱导。然而,在亚毒性的锰剂量以及较长的孵育时间下无法观察到这种效应。对于锰诱导的氧化应激,缺失突变体没有表现出超敏反应。总而言之,这项研究描述了蠕虫的特征,以模拟 PAR 抑制,并解决了基因操纵蠕虫中锰诱导的氧化应激的后果。
更新日期:2019-09-14
中文翻译:

聚(ADP-核糖)聚合酶在锰暴露的秀丽隐杆线虫中的作用。
背景和目的 当超过稳态范围时,锰 (Mn) 可能会引起神经毒性,这是几种神经系统疾病的病理生理学特征。尽管其神经毒性的潜在机制尚不清楚,但锰诱导的氧化应激有助于疾病病因学。氧化应激引起的 DNA 损伤可能进一步引发 DNA 损伤诱导的聚(ADP-核糖基)化(PARylation)失调,这对于神经元稳态至关重要。因此,本研究旨在评估遗传可追溯的体内模型秀丽隐杆线虫中 PARylation 的作用以及 pme-1 或 pme-2(PARP1 和 PARP2 的直系同源物)丢失在锰诱导的毒性中的后果。方法开发了一种特异且灵敏的同位素稀释液相色谱-串联质谱 (LC-MS/MS) 方法来定量蠕虫中的 PARylation。接下来监测 PAR 水平,在野生型线虫和 pme 缺失突变体中研究了 pme-1 和 pme-2 基因表达以及 Mn 诱导的氧化应激。结果和结论 虽然 Mn 未能在野生型蠕虫中诱导 PARylation,但由于 pme-1 缺失突变体中 pme-2 基因表达增加,有毒剂量的 Mn 导致 pme-1 缺陷型蠕虫中 PAR 诱导。然而,在亚毒性的锰剂量以及较长的孵育时间下无法观察到这种效应。对于锰诱导的氧化应激,缺失突变体没有表现出超敏反应。总而言之,这项研究描述了蠕虫的特征,以模拟 PAR 抑制,并解决了基因操纵蠕虫中锰诱导的氧化应激的后果。










































 京公网安备 11010802027423号
京公网安备 11010802027423号