Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antibiotic or silver versus standard ventriculoperitoneal shunts (BASICS): a multicentre, single-blinded, randomised trial and economic evaluation.
The Lancet ( IF 98.4 ) Pub Date : 2019-09-12 , DOI: 10.1016/s0140-6736(19)31603-4 Conor L Mallucci 1 , Michael D Jenkinson 2 , Elizabeth J Conroy 3 , John C Hartley 4 , Michaela Brown 3 , Joanne Dalton 3 , Tom Kearns 3 , Tracy Moitt 3 , Michael J Griffiths 5 , Giovanna Culeddu 6 , Tom Solomon 7 , Dyfrig Hughes 6 , Carrol Gamble 3 ,
The Lancet ( IF 98.4 ) Pub Date : 2019-09-12 , DOI: 10.1016/s0140-6736(19)31603-4 Conor L Mallucci 1 , Michael D Jenkinson 2 , Elizabeth J Conroy 3 , John C Hartley 4 , Michaela Brown 3 , Joanne Dalton 3 , Tom Kearns 3 , Tracy Moitt 3 , Michael J Griffiths 5 , Giovanna Culeddu 6 , Tom Solomon 7 , Dyfrig Hughes 6 , Carrol Gamble 3 ,
Affiliation
|
BACKGROUND
Insertion of a ventriculoperitoneal shunt for hydrocephalus is one of the commonest neurosurgical procedures worldwide. Infection of the implanted shunt affects up to 15% of these patients, resulting in prolonged hospital treatment, multiple surgeries, and reduced cognition and quality of life. Our aim was to determine the clinical and cost-effectiveness of antibiotic (rifampicin and clindamycin) or silver shunts compared with standard shunts at reducing infection.
METHODS
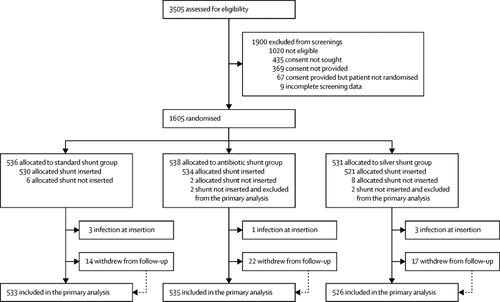
In this parallel, multicentre, single-blind, randomised controlled trial, we included patients with hydrocephalus of any aetiology undergoing insertion of their first ventriculoperitoneal shunt irrespective of age at 21 regional adult and paediatric neurosurgery centres in the UK and Ireland. Patients were randomly assigned (1:1:1 in random permuted blocks of three or six) to receive standard shunts (standard shunt group), antibiotic-impregnated (0·15% clindamycin and 0·054% rifampicin; antibiotic shunt group), or silver-impregnated shunts (silver shunt group) through a randomisation sequence generated by an independent statistician. All patients and investigators who recorded and analysed the data were masked for group assignment, which was only disclosed to the neurosurgical staff at the time of operation. Participants receiving a shunt without evidence of infection at the time of insertion were followed up for at least 6 months and a maximum of 2 years. The primary outcome was time to shunt failure due the infection and was analysed with Fine and Gray survival regression models for competing risk by intention to treat. This trial is registered with ISRCTN 49474281.
FINDINGS
Between June 26, 2013, and Oct 9, 2017, we assessed 3505 patients, of whom 1605 aged up to 91 years were randomly assigned to receive either a standard shunt (n=536), an antibiotic-impregnated shunt (n=538), or a silver shunt (n=531). 1594 had a shunt inserted without evidence of infection at the time of insertion (533 in the standard shunt group, 535 in the antibiotic shunt group, and 526 in the silver shunt group) and were followed up for a median of 22 months (IQR 10-24; 53 withdrew from follow-up). 32 (6%) of 533 evaluable patients in the standard shunt group had a shunt revision for infection, compared with 12 (2%) of 535 evaluable patients in the antibiotic shunt group (cause-specific hazard ratio [csHR] 0·38, 97·5% CI 0·18-0·80, p=0·0038) and 31 (6%) of 526 patients in the silver shunt group (0·99, 0·56-1·74, p=0·96). 135 (25%) patients in the standard shunt group, 127 (23%) in the antibiotic shunt group, and 134 (36%) in the silver shunt group had adverse events, which were not life-threatening and were mostly related to valve or catheter function.
INTERPRETATION
The BASICS trial provides evidence to support the adoption of antibiotic shunts in UK patients who are having their first ventriculoperitoneal shunt insertion. This practice will benefit patients of all ages by reducing the risk and harm of shunt infection.
FUNDING
UK National Institute for Health Research Health Technology Assessment programme.
中文翻译:

抗生素或银与标准脑室腹腔分流术 (BASICS):一项多中心、单盲、随机试验和经济评估。
背景技术为脑积水插入脑室腹腔分流术是世界范围内最常见的神经外科手术之一。植入分流器的感染影响了这些患者中多达 15%,导致住院治疗时间延长、多次手术以及认知和生活质量下降。我们的目的是确定抗生素(利福平和克林霉素)或银分流术与标准分流术相比在减少感染方面的临床和成本效益。方法 在这项平行、多中心、单盲、随机对照试验中,我们纳入了在英国和爱尔兰的 21 个区域性成人和儿童神经外科中心接受首次脑室腹腔分流术的任何病因的脑积水患者,无论年龄大小。患者被随机分配(1:1: 1 个随机排列的块,每块三个或六个)接受标准分流(标准分流组)、抗生素浸渍(0·15% 克林霉素和 0·054% 利福平;抗生素分流组)或银浸渍分流(银分流组) ) 通过独立统计学家生成的随机化序列。所有记录和分析数据的患者和研究人员都被设盲以进行分组,仅在手术时向神经外科工作人员披露。对在插入时没有感染证据而接受分流术的参与者进行至少 6 个月、最长 2 年的随访。主要结果是分流因感染失败的时间,并使用 Fine 和 Gray 生存回归模型分析意向治疗的竞争风险。该试验已在 ISRCTN 49474281 注册。2013 年 6 月 26 日至 2017 年 10 月 9 日期间,我们评估了 3505 名患者,其中 1605 名年龄不超过 91 岁的患者被随机分配接受标准分流术 (n=536)、抗生素浸渍分流术 (n=538) ), 或银分流器 (n=531)。1594 名在插入时没有感染证据的分流器(标准分流器组 533 名、抗生素分流器组 535 名和银分流器组 526 名)进行了中位随访 22 个月(IQR 10 -24;53 人退出随访)。标准分流组 533 名可评估患者中有 32 名 (6%) 因感染进行了分流术翻修,而抗生素分流组 535 名可评估患者中有 12 名 (2%) 进行了分流术翻修(病因特异性风险比 [csHR] 0·38, 97·5% CI 0·18-0·80, p=0·0038) 和银分流术组 526 名患者中的 31 (6%) (0·99, 0·56-1·74, p=0· 96). 标准分流组 135 名 (25%) 患者、抗生素分流组 127 名 (23%) 和银分流组 134 名 (36%) 患者出现不良事件,这些不良事件不危及生命,主要与瓣膜相关或导管功能。解释 BASICS 试验提供的证据支持对首次脑室腹腔分流术插入的英国患者采用抗生素分流术。这种做法可降低分流感染的风险和危害,从而使所有年龄段的患者受益。资助英国国家卫生研究所卫生技术评估计划。解释 BASICS 试验提供的证据支持对首次脑室腹腔分流术插入的英国患者采用抗生素分流术。这种做法可降低分流感染的风险和危害,从而使所有年龄段的患者受益。资助英国国家卫生研究所卫生技术评估计划。解释 BASICS 试验提供的证据支持对首次脑室腹腔分流术插入的英国患者采用抗生素分流术。这种做法可降低分流感染的风险和危害,从而使所有年龄段的患者受益。资助英国国家卫生研究所卫生技术评估计划。
更新日期:2019-10-25
中文翻译:

抗生素或银与标准脑室腹腔分流术 (BASICS):一项多中心、单盲、随机试验和经济评估。
背景技术为脑积水插入脑室腹腔分流术是世界范围内最常见的神经外科手术之一。植入分流器的感染影响了这些患者中多达 15%,导致住院治疗时间延长、多次手术以及认知和生活质量下降。我们的目的是确定抗生素(利福平和克林霉素)或银分流术与标准分流术相比在减少感染方面的临床和成本效益。方法 在这项平行、多中心、单盲、随机对照试验中,我们纳入了在英国和爱尔兰的 21 个区域性成人和儿童神经外科中心接受首次脑室腹腔分流术的任何病因的脑积水患者,无论年龄大小。患者被随机分配(1:1: 1 个随机排列的块,每块三个或六个)接受标准分流(标准分流组)、抗生素浸渍(0·15% 克林霉素和 0·054% 利福平;抗生素分流组)或银浸渍分流(银分流组) ) 通过独立统计学家生成的随机化序列。所有记录和分析数据的患者和研究人员都被设盲以进行分组,仅在手术时向神经外科工作人员披露。对在插入时没有感染证据而接受分流术的参与者进行至少 6 个月、最长 2 年的随访。主要结果是分流因感染失败的时间,并使用 Fine 和 Gray 生存回归模型分析意向治疗的竞争风险。该试验已在 ISRCTN 49474281 注册。2013 年 6 月 26 日至 2017 年 10 月 9 日期间,我们评估了 3505 名患者,其中 1605 名年龄不超过 91 岁的患者被随机分配接受标准分流术 (n=536)、抗生素浸渍分流术 (n=538) ), 或银分流器 (n=531)。1594 名在插入时没有感染证据的分流器(标准分流器组 533 名、抗生素分流器组 535 名和银分流器组 526 名)进行了中位随访 22 个月(IQR 10 -24;53 人退出随访)。标准分流组 533 名可评估患者中有 32 名 (6%) 因感染进行了分流术翻修,而抗生素分流组 535 名可评估患者中有 12 名 (2%) 进行了分流术翻修(病因特异性风险比 [csHR] 0·38, 97·5% CI 0·18-0·80, p=0·0038) 和银分流术组 526 名患者中的 31 (6%) (0·99, 0·56-1·74, p=0· 96). 标准分流组 135 名 (25%) 患者、抗生素分流组 127 名 (23%) 和银分流组 134 名 (36%) 患者出现不良事件,这些不良事件不危及生命,主要与瓣膜相关或导管功能。解释 BASICS 试验提供的证据支持对首次脑室腹腔分流术插入的英国患者采用抗生素分流术。这种做法可降低分流感染的风险和危害,从而使所有年龄段的患者受益。资助英国国家卫生研究所卫生技术评估计划。解释 BASICS 试验提供的证据支持对首次脑室腹腔分流术插入的英国患者采用抗生素分流术。这种做法可降低分流感染的风险和危害,从而使所有年龄段的患者受益。资助英国国家卫生研究所卫生技术评估计划。解释 BASICS 试验提供的证据支持对首次脑室腹腔分流术插入的英国患者采用抗生素分流术。这种做法可降低分流感染的风险和危害,从而使所有年龄段的患者受益。资助英国国家卫生研究所卫生技术评估计划。











































 京公网安备 11010802027423号
京公网安备 11010802027423号