Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): a large, randomised, double-blind, placebo-controlled, phase 2 study.
The Lancet ( IF 98.4 ) Pub Date : 2019-09-12 , DOI: 10.1016/s0140-6736(19)31793-3 R Sharon Chinthrajah 1 , Natasha Purington 2 , Sandra Andorf 1 , Andrew Long 1 , Katherine L O'Laughlin 1 , Shu Chen Lyu 1 , Monali Manohar 1 , Scott D Boyd 3 , Robert Tibshirani 4 , Holden Maecker 5 , Marshall Plaut 6 , Kaori Mukai 3 , Mindy Tsai 3 , Manisha Desai 7 , Stephen J Galli 8 , Kari C Nadeau 1
The Lancet ( IF 98.4 ) Pub Date : 2019-09-12 , DOI: 10.1016/s0140-6736(19)31793-3 R Sharon Chinthrajah 1 , Natasha Purington 2 , Sandra Andorf 1 , Andrew Long 1 , Katherine L O'Laughlin 1 , Shu Chen Lyu 1 , Monali Manohar 1 , Scott D Boyd 3 , Robert Tibshirani 4 , Holden Maecker 5 , Marshall Plaut 6 , Kaori Mukai 3 , Mindy Tsai 3 , Manisha Desai 7 , Stephen J Galli 8 , Kari C Nadeau 1
Affiliation
|
BACKGROUND
Dietary avoidance is recommended for peanut allergies. We evaluated the sustained effects of peanut allergy oral immunotherapy (OIT) in a randomised long-term study in adults and children.
METHODS
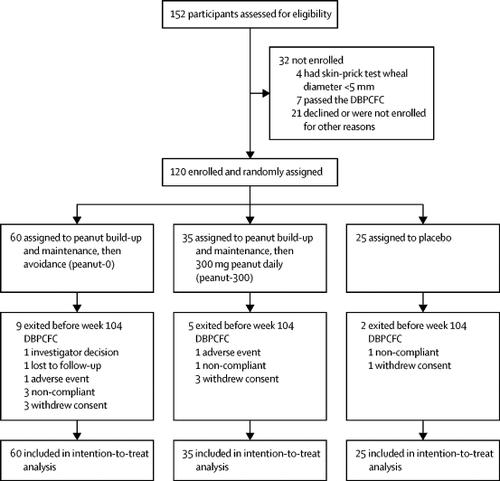
In this randomised, double-blind, placebo-controlled, phase 2 study, we enrolled participants at the Sean N Parker Center for Allergy and Asthma Research at Stanford University (Stanford, CA, USA) with peanut allergy aged 7-55 years with a positive result from a double-blind, placebo-controlled, food challenge (DBPCFC; ≤500 mg of peanut protein), a positive skin-prick test (SPT) result (≥5 mm wheal diameter above the negative control), and peanut-specific immunoglobulin (Ig)E concentration of more than 4 kU/L. Participants were randomly assigned (2·4:1·4:1) in a two-by-two block design via a computerised system to be built up and maintained on 4000 mg peanut protein through to week 104 then discontinued on peanut (peanut-0 group), to be built up and maintained on 4000 mg peanut protein through to week 104 then to ingest 300 mg peanut protein daily (peanut-300 group) for 52 weeks, or to receive oat flour (placebo group). DBPCFCs to 4000 mg peanut protein were done at baseline and weeks 104, 117, 130, 143, and 156. The pharmacist assigned treatment on the basis of a randomised computer list. Peanut or placebo (oat) flour was administered orally and participants and the study team were masked throughout by use of oat flour that was similar in look and feel to the peanut flour and nose clips, as tolerated, to mask taste. The statistician was also masked. The primary endpoint was the proportion of participants who passed DBPCFCs to a cumulative dose of 4000 mg at both 104 and 117 weeks. The primary efficacy analysis was done in the intention-to-treat population. Safety was assessed in the intention-to-treat population. This trial is registered at ClinicalTrials.gov, NCT02103270.
FINDINGS
Between April 15, 2014, and March 2, 2016, of 152 individuals assessed, we enrolled 120 participants, who were randomly assigned to the peanut-0 (n=60), peanut-300 (n=35), and placebo groups (n=25). 21 (35%) of peanut-0 group participants and one (4%) placebo group participant passed the 4000 mg challenge at both 104 and 117 weeks (odds ratio [OR] 12·7, 95% CI 1·8-554·8; p=0·0024). Over the entire study, the most common adverse events were mild gastrointestinal symptoms, which were seen in 90 of 120 patients (50/60 in the peanut-0 group, 29/35 in the peanut-300 group, and 11/25 in the placebo group) and skin disorders, which were seen in 50/120 patients (26/60 in the peanut-0 group, 15/35 in the peanut-300 group, and 9/25 in the placebo group). Adverse events decreased over time in all groups. Two participants in the peanut groups had serious adverse events during the 3-year study. In the peanut-0 group, in which eight (13%) of 60 participants passed DBPCFCs at week 156, higher baseline peanut-specific IgG4 to IgE ratio and lower Ara h 2 IgE and basophil activation responses were associated with sustained unresponsiveness. No treatment-related deaths occurred.
INTERPRETATION
Our study suggests that peanut OIT could desensitise individuals with peanut allergy to 4000 mg peanut protein but discontinuation, or even reduction to 300 mg daily, could increase the likelihood of regaining clinical reactivity to peanut. Since baseline blood tests correlated with week 117 treatment outcomes, this study might aid in optimal patient selection for this therapy.
FUNDING
National Institute of Allergy and Infectious Diseases.
中文翻译:

花生过敏症口服免疫疗法的持续成果(POISED研究):一项大型,随机,双盲,安慰剂对照的2期研究。
背景技术建议饮食避免花生过敏。在一项针对成人和儿童的随机长期研究中,我们评估了花生过敏口服免疫疗法(OIT)的持续作用。方法在这项随机,双盲,安慰剂对照的2期研究中,我们招募了美国斯坦福大学Sean N Parker过敏和哮喘研究中心的参与者,研究对象是7-55岁的花生过敏,双盲,安慰剂对照食物挑战(DBPCFC;≤500 mg花生蛋白)呈阳性结果,皮肤点刺试验(SPT)呈阳性结果(比阴性对照高≥5 mm的烟团直径),以及花生特异性免疫球蛋白(Ig)E的浓度超过4 kU / L。参与者被随机分配(2·4:1·4:1)通过计算机系统以2乘2的方式进行设计,以4000 mg的花生蛋白构建并维持至第104周,然后以花生(花生0组)停产,以4000 mg的构建并维持花生蛋白直到第104周,然后每天摄取300 mg花生蛋白(peanut-300组)持续52周,或接受燕麦粉(安慰剂组)。在基线以及第104、117、130、143和156周时,对4000 mg花生蛋白进行了DBPCFCs处理。药剂师根据随机计算机列表分配了治疗方法。口服花生或安慰剂(燕麦)粉,并通过使用与花生粉和鼻夹相似的外观和感觉遮盖味道的燕麦粉对参与者和研究团队进行全面遮盖。这位统计学家也被掩盖了。主要终点是在104周和117周通过DBPCFCs累积剂量达到4000 mg的参与者比例。主要疗效分析是在意向性治疗人群中进行的。在意向治疗人群中评估安全性。该试验已在ClinicalTrials.gov上注册,NCT02103270。结果在2014年4月15日至2016年3月2日期间,我们对152位参与者进行了评估,共招募了120位参与者,他们被随机分为花生0(n = 60),花生300(n = 35)和安慰剂组(n = 25)。花生0组参与者中有21名(35%)和安慰剂组参与者中有1名(4%)在104和117周时均通过了4000 mg的挑战(赔率[OR] 12·7、95%CI 1·8-554· 8; p = 0·0024)。在整个研究中,最常见的不良事件是轻度的胃肠道症状,120例患者中有90例(花生0组为50/60,花生300组为29/35,安慰剂组为11/25)和皮肤疾病,50/120例中(花生0组为26/60,花生300组为15/35,安慰剂组为9/25)。所有组的不良事件均随着时间而减少。在为期3年的研究中,花生组的两名参与者出现了严重的不良事件。在花生0组中,有60名参与者中的8名(13%)在第156周通过了DBPCFC,较高的基线花生特异性IgG4与IgE比率以及较低的Ara h 2 IgE和嗜碱性粒细胞活化反应与持续的无反应性相关。没有发生与治疗有关的死亡。解释我们的研究表明,花生OIT可以使对花生过敏的个体对4000 mg花生蛋白不敏感,但停用该蛋白,甚至减少到每天300毫克,可能会增加恢复对花生的临床反应性的可能性。由于基线血液测试与第117周的治疗结果相关,因此本研究可能有助于选择该疗法的最佳患者。资助国家过敏和传染病研究所。
更新日期:2019-10-17
中文翻译:

花生过敏症口服免疫疗法的持续成果(POISED研究):一项大型,随机,双盲,安慰剂对照的2期研究。
背景技术建议饮食避免花生过敏。在一项针对成人和儿童的随机长期研究中,我们评估了花生过敏口服免疫疗法(OIT)的持续作用。方法在这项随机,双盲,安慰剂对照的2期研究中,我们招募了美国斯坦福大学Sean N Parker过敏和哮喘研究中心的参与者,研究对象是7-55岁的花生过敏,双盲,安慰剂对照食物挑战(DBPCFC;≤500 mg花生蛋白)呈阳性结果,皮肤点刺试验(SPT)呈阳性结果(比阴性对照高≥5 mm的烟团直径),以及花生特异性免疫球蛋白(Ig)E的浓度超过4 kU / L。参与者被随机分配(2·4:1·4:1)通过计算机系统以2乘2的方式进行设计,以4000 mg的花生蛋白构建并维持至第104周,然后以花生(花生0组)停产,以4000 mg的构建并维持花生蛋白直到第104周,然后每天摄取300 mg花生蛋白(peanut-300组)持续52周,或接受燕麦粉(安慰剂组)。在基线以及第104、117、130、143和156周时,对4000 mg花生蛋白进行了DBPCFCs处理。药剂师根据随机计算机列表分配了治疗方法。口服花生或安慰剂(燕麦)粉,并通过使用与花生粉和鼻夹相似的外观和感觉遮盖味道的燕麦粉对参与者和研究团队进行全面遮盖。这位统计学家也被掩盖了。主要终点是在104周和117周通过DBPCFCs累积剂量达到4000 mg的参与者比例。主要疗效分析是在意向性治疗人群中进行的。在意向治疗人群中评估安全性。该试验已在ClinicalTrials.gov上注册,NCT02103270。结果在2014年4月15日至2016年3月2日期间,我们对152位参与者进行了评估,共招募了120位参与者,他们被随机分为花生0(n = 60),花生300(n = 35)和安慰剂组(n = 25)。花生0组参与者中有21名(35%)和安慰剂组参与者中有1名(4%)在104和117周时均通过了4000 mg的挑战(赔率[OR] 12·7、95%CI 1·8-554· 8; p = 0·0024)。在整个研究中,最常见的不良事件是轻度的胃肠道症状,120例患者中有90例(花生0组为50/60,花生300组为29/35,安慰剂组为11/25)和皮肤疾病,50/120例中(花生0组为26/60,花生300组为15/35,安慰剂组为9/25)。所有组的不良事件均随着时间而减少。在为期3年的研究中,花生组的两名参与者出现了严重的不良事件。在花生0组中,有60名参与者中的8名(13%)在第156周通过了DBPCFC,较高的基线花生特异性IgG4与IgE比率以及较低的Ara h 2 IgE和嗜碱性粒细胞活化反应与持续的无反应性相关。没有发生与治疗有关的死亡。解释我们的研究表明,花生OIT可以使对花生过敏的个体对4000 mg花生蛋白不敏感,但停用该蛋白,甚至减少到每天300毫克,可能会增加恢复对花生的临床反应性的可能性。由于基线血液测试与第117周的治疗结果相关,因此本研究可能有助于选择该疗法的最佳患者。资助国家过敏和传染病研究所。









































 京公网安备 11010802027423号
京公网安备 11010802027423号