当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselective Synthesis of 3,4-Dihydropyran-3-carboxamides with in Vitro Anti-inflammatory Activity.
ACS Combinatorial Science Pub Date : 2019-09-11 , DOI: 10.1021/acscombsci.9b00050 Li-Yan Zeng 1 , Baomin Xi 1 , Kaiqi Huang 1 , Jingjie Bi 1 , Lan Wei 1 , Chun Cai 2 , Shuwen Liu 1, 3
ACS Combinatorial Science Pub Date : 2019-09-11 , DOI: 10.1021/acscombsci.9b00050 Li-Yan Zeng 1 , Baomin Xi 1 , Kaiqi Huang 1 , Jingjie Bi 1 , Lan Wei 1 , Chun Cai 2 , Shuwen Liu 1, 3
Affiliation
|
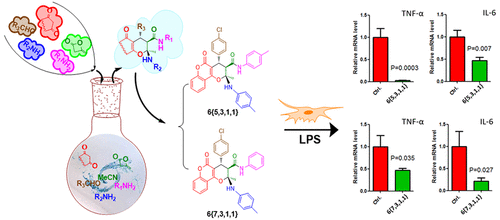
A versatile and economical reaction of diketene (1), aryl amines 2, cyclic 1,3-diketones 3, primary amines 4, and aryl aldehydes 5 was explored to synthesize 3,4-dihydropyran-3-carboxamide derivatives under mild conditions. Three stereogenic centers are generated in the products, and the structure of the major diastereomer of 6{1,1,3,1} was identified by X-ray diffraction and 2D NMR spectroscopy. The scope and limitation investigation provided two series of (2S,3R,4S)-chromene-3-carboxamides in good to excellent yields with high diastereoselectivity. Two products, 6{5,3,1,1} and 6{7,3,1,1}, exhibited in vitro anti-inflammatory activity with significant inhibition of pro-inflammatory cytokine IL-6 and TNF-α expression in lipopolysaccharide (LPS)-treated Raw 264.7 cells.
中文翻译:

具有体外抗炎活性的3,4-二氢吡喃-3-羧酰胺的非对映选择性合成。
探索了在温和条件下,双烯酮(1),芳基胺2,环状1,3-二酮3,伯胺4和芳基醛5的一种通用且经济的反应,可以合成3,4-二氢吡喃-3-甲酰胺衍生物。产品中生成了三个立体异构中心,并通过X射线衍射和2D NMR光谱鉴定了6 {1,1,3,1}的主要非对映异构体的结构。范围和局限性研究提供了两个系列的(2S,3R,4S)-亚甲基-3-羧酰胺,具有良好的收率和优异的非对映选择性。两种产品6 {5,3,1,1}和6 {7,3,1,1}表现出体外抗炎活性,并显着抑制脂多糖中促炎性细胞因子IL-6和TNF-α的表达(LPS)处理的Raw 264.7细胞。
更新日期:2019-09-11
中文翻译:

具有体外抗炎活性的3,4-二氢吡喃-3-羧酰胺的非对映选择性合成。
探索了在温和条件下,双烯酮(1),芳基胺2,环状1,3-二酮3,伯胺4和芳基醛5的一种通用且经济的反应,可以合成3,4-二氢吡喃-3-甲酰胺衍生物。产品中生成了三个立体异构中心,并通过X射线衍射和2D NMR光谱鉴定了6 {1,1,3,1}的主要非对映异构体的结构。范围和局限性研究提供了两个系列的(2S,3R,4S)-亚甲基-3-羧酰胺,具有良好的收率和优异的非对映选择性。两种产品6 {5,3,1,1}和6 {7,3,1,1}表现出体外抗炎活性,并显着抑制脂多糖中促炎性细胞因子IL-6和TNF-α的表达(LPS)处理的Raw 264.7细胞。











































 京公网安备 11010802027423号
京公网安备 11010802027423号