Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Corticosteroid-Sparing Treatment With Mycophenolate Mofetil vs Methotrexate on Inflammation in Patients With Uveitis
JAMA ( IF 63.1 ) Pub Date : 2019-09-10 , DOI: 10.1001/jama.2019.12618 S R Rathinam 1 , John A Gonzales 2, 3 , Radhika Thundikandy 1 , Anuradha Kanakath 4 , S Bala Murugan 5 , R Vedhanayaki 1 , Lyndell L Lim 6 , Eric B Suhler 7, 8, 9 , Hassan A Al-Dhibi 10 , Thuy Doan 2, 3 , Jeremy D Keenan 2, 3 , Maya M Rao 2 , Caleb D Ebert 2 , Hieu H Nguyen 2 , Eric Kim 2 , Travis C Porco 2, 3, 11 , Nisha R Acharya 2, 3, 11 ,
JAMA ( IF 63.1 ) Pub Date : 2019-09-10 , DOI: 10.1001/jama.2019.12618 S R Rathinam 1 , John A Gonzales 2, 3 , Radhika Thundikandy 1 , Anuradha Kanakath 4 , S Bala Murugan 5 , R Vedhanayaki 1 , Lyndell L Lim 6 , Eric B Suhler 7, 8, 9 , Hassan A Al-Dhibi 10 , Thuy Doan 2, 3 , Jeremy D Keenan 2, 3 , Maya M Rao 2 , Caleb D Ebert 2 , Hieu H Nguyen 2 , Eric Kim 2 , Travis C Porco 2, 3, 11 , Nisha R Acharya 2, 3, 11 ,
Affiliation
|
Importance
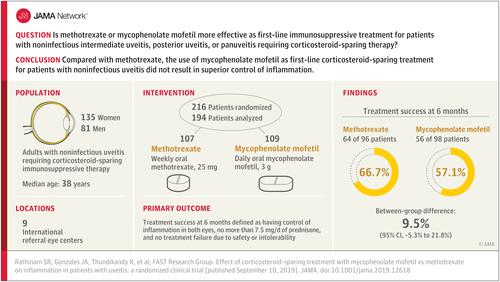
Methotrexate and mycophenolate mofetil are commonly used immunomodulatory therapies for achieving corticosteroid-sparing control of noninfectious uveitis, but there is uncertainty about which drug is more effective. Objective
To compare the effect of methotrexate and mycophenolate for achieving corticosteroid-sparing control of noninfectious intermediate uveitis, posterior uveitis, and panuveitis. Design, Setting, and Participants
The First-line Antimetabolites as Steroid-sparing Treatment (FAST) uveitis trial screened 265 adults with noninfectious uveitis requiring corticosteroid-sparing immunosuppressive therapy from 9 referral eye centers in India, the United States, Australia, Saudi Arabia, and Mexico between August 22, 2013, and August 16, 2017. Follow-up ended on August 20, 2018. Interventions
Patients were randomized to receive oral methotrexate, 25 mg weekly (n = 107), or oral mycophenolate mofetil, 3 g daily (n = 109). Main Outcomes and Measures
The primary outcome was treatment success at 6 months, which was defined as having control of inflammation in both eyes, no more than 7.5 mg prednisone daily and less than or equal to 2 drops of prednisolone acetate 1%, and no treatment failure due to safety or intolerability. Patients underwent follow-up to 12 months while receiving the same treatment or switched to the other antimetabolite, depending on their 6-month outcome. Results
Among 216 patients who were randomized (median age, 38 years; 135 (62.5%) women), 194 (89.8%) completed follow-up through 6 months. Treatment success occurred in 64 (66.7%) patients in the methotrexate group vs 56 (57.1%) in the mycophenolate group (difference, 9.5% [95% CI, -5.3% to 21.8%]; odds ratio [OR], 1.50 [95% CI, 0.81 to 2.81]; P = .20). Among patients with posterior uveitis or panuveitis, treatment success was achieved in 58 (74.4%) in the methotrexate group vs 42 (55.3%) in the mycophenolate group (difference, 19.1% [95% CI, 3.6% to 30.6%]; OR, 2.35 [95% CI, 1.16 to 4.90]; P = .02); whereas among patients with intermediate uveitis treatment success occurred in 6 (33.3%) in the methotrexate group vs 14 (63.6%) in the mycophenolate group (difference, -30.3% [95% CI, -51.6% to 1.1%]; OR, 0.29 [95% CI, 0.08 to 1.05]; P = .07; P for interaction = .004). Elevated liver enzymes were the most common nonserious laboratory adverse event, occurring in 14 patients (13.0%) in the methotrexate group and 8 patients (7.4%) in the mycophenolate group. Conclusions and Relevance
Among adults with noninfectious uveitis, the use of mycophenolate mofetil compared with methotrexate as first-line corticosteroid-sparing treatment did not result in superior control of inflammation. Further research is needed to determine if either drug is more effective based on the anatomical subtype of uveitis. Trial Registration
ClinicalTrials.gov Identifier: NCT01829295.
中文翻译:

吗替麦考酚酯与甲氨蝶呤的皮质类固醇保留治疗对葡萄膜炎患者炎症的影响
重要性 甲氨蝶呤和吗替麦考酚酯是常用的免疫调节疗法,用于实现非感染性葡萄膜炎的少皮质类固醇控制,但尚不确定哪种药物更有效。目的比较甲氨蝶呤与霉酚酸酯对非感染性中间葡萄膜炎、后葡萄膜炎和全葡萄膜炎的不使用糖皮质激素控制的效果。设计、设置和参与者 一线抗代谢药物作为类固醇保留治疗 (FAST) 葡萄膜炎试验筛选了来自印度、美国、澳大利亚、沙特阿拉伯的 9 个转诊眼科中心的 265 名需要保留皮质类固醇免疫抑制治疗的非感染性葡萄膜炎成人, 2013 年 8 月 22 日至 2017 年 8 月 16 日期间在墨西哥和墨西哥。随访于 2018 年 8 月 20 日结束。干预 患者随机接受口服甲氨蝶呤,每周 25 毫克(n = 107),或口服霉酚酸酯,每天 3 克(n = 109)。主要结果和措施 主要结果是 6 个月时的治疗成功,定义为双眼炎症得到控制,每天不超过 7.5 mg 泼尼松和小于或等于 2 滴 1% 醋酸泼尼松龙,并且没有治疗由于安全性或不可耐受性而导致的故障。患者在接受相同治疗的同时接受了长达 12 个月的随访,或者根据 6 个月的结果改用其他抗代谢药物。结果 在随机分组的 216 名患者(中位年龄,38 岁;135 名(62.5%)女性)中,194 名(89.8%)完成了 6 个月的随访。甲氨蝶呤组有 64 名 (66.7%) 患者和 56 名 (57. 1%)在霉酚酸酯组(差异,9.5% [95% CI,-5.3% 至 21.8%];优势比 [OR],1.50 [95% CI,0.81 至 2.81];P = .20)。在后葡萄膜炎或全葡萄膜炎患者中,甲氨蝶呤组 58 名 (74.4%) 与霉酚酸酯组 42 名 (55.3%) 取得治疗成功(差异,19.1% [95% CI,3.6% 至 30.6%];或, 2.35 [95% CI,1.16 至 4.90];P = .02);而在中间葡萄膜炎患者中,甲氨蝶呤组 6 例(33.3%)与霉酚酸酯组 14 例(63.6%)(差异,-30.3% [95% CI,-51.6% 至 1.1%];OR, 0.29 [95% CI,0.08 至 1.05];P = .07;交互作用的 P = .004)。肝酶升高是最常见的非严重实验室不良事件,发生在甲氨蝶呤组 14 名患者 (13.0%) 和霉酚酸酯组 8 名患者 (7.4%) 中。结论和相关性 在患有非感染性葡萄膜炎的成人中,与甲氨蝶呤相比,使用霉酚酸酯作为一线保留皮质类固醇的治疗并不能更好地控制炎症。需要进一步的研究来根据葡萄膜炎的解剖亚型确定这两种药物是否更有效。试验注册 ClinicalTrials.gov 标识符:NCT01829295。
更新日期:2019-09-10
中文翻译:

吗替麦考酚酯与甲氨蝶呤的皮质类固醇保留治疗对葡萄膜炎患者炎症的影响
重要性 甲氨蝶呤和吗替麦考酚酯是常用的免疫调节疗法,用于实现非感染性葡萄膜炎的少皮质类固醇控制,但尚不确定哪种药物更有效。目的比较甲氨蝶呤与霉酚酸酯对非感染性中间葡萄膜炎、后葡萄膜炎和全葡萄膜炎的不使用糖皮质激素控制的效果。设计、设置和参与者 一线抗代谢药物作为类固醇保留治疗 (FAST) 葡萄膜炎试验筛选了来自印度、美国、澳大利亚、沙特阿拉伯的 9 个转诊眼科中心的 265 名需要保留皮质类固醇免疫抑制治疗的非感染性葡萄膜炎成人, 2013 年 8 月 22 日至 2017 年 8 月 16 日期间在墨西哥和墨西哥。随访于 2018 年 8 月 20 日结束。干预 患者随机接受口服甲氨蝶呤,每周 25 毫克(n = 107),或口服霉酚酸酯,每天 3 克(n = 109)。主要结果和措施 主要结果是 6 个月时的治疗成功,定义为双眼炎症得到控制,每天不超过 7.5 mg 泼尼松和小于或等于 2 滴 1% 醋酸泼尼松龙,并且没有治疗由于安全性或不可耐受性而导致的故障。患者在接受相同治疗的同时接受了长达 12 个月的随访,或者根据 6 个月的结果改用其他抗代谢药物。结果 在随机分组的 216 名患者(中位年龄,38 岁;135 名(62.5%)女性)中,194 名(89.8%)完成了 6 个月的随访。甲氨蝶呤组有 64 名 (66.7%) 患者和 56 名 (57. 1%)在霉酚酸酯组(差异,9.5% [95% CI,-5.3% 至 21.8%];优势比 [OR],1.50 [95% CI,0.81 至 2.81];P = .20)。在后葡萄膜炎或全葡萄膜炎患者中,甲氨蝶呤组 58 名 (74.4%) 与霉酚酸酯组 42 名 (55.3%) 取得治疗成功(差异,19.1% [95% CI,3.6% 至 30.6%];或, 2.35 [95% CI,1.16 至 4.90];P = .02);而在中间葡萄膜炎患者中,甲氨蝶呤组 6 例(33.3%)与霉酚酸酯组 14 例(63.6%)(差异,-30.3% [95% CI,-51.6% 至 1.1%];OR, 0.29 [95% CI,0.08 至 1.05];P = .07;交互作用的 P = .004)。肝酶升高是最常见的非严重实验室不良事件,发生在甲氨蝶呤组 14 名患者 (13.0%) 和霉酚酸酯组 8 名患者 (7.4%) 中。结论和相关性 在患有非感染性葡萄膜炎的成人中,与甲氨蝶呤相比,使用霉酚酸酯作为一线保留皮质类固醇的治疗并不能更好地控制炎症。需要进一步的研究来根据葡萄膜炎的解剖亚型确定这两种药物是否更有效。试验注册 ClinicalTrials.gov 标识符:NCT01829295。











































 京公网安备 11010802027423号
京公网安备 11010802027423号