The Lancet Respiratory Medicine ( IF 38.7 ) Pub Date : 2019-09-09 , DOI: 10.1016/s2213-2600(19)30269-3 Natalie Walker 1 , Varsha Parag 1 , Marjolein Verbiest 2 , George Laking 3 , Murray Laugesen 4 , Christopher Bullen 1
|
Background
Combination nicotine replacement therapy shows additive cessation benefits. We aimed to find out the effectiveness of combining nicotine patches with an e-cigarette (with and without nicotine) on six-month smoking abstinence.
Methods
We did a pragmatic, three-arm, parallel-group trial in New Zealand in adult smokers who were e-cigarette naive and motivated to quit smoking. Participants were recruited from the general population using national media advertising. Participants were randomly assigned (1:4:4), with the use of stratified block randomisation, to receive 14 weeks (2 weeks before the agreed quit date) of 21 mg, 24h nicotine patches, patches plus an 18 mg/L nicotine e-cigarette, or patches plus a nicotine-free e-cigarette. We advised participants to use one patch daily, with e-cigarette use as and when necessary or desired. Participants and researchers were masked to e-liquid nicotine content. We offered 6 weeks of telephone-delivered behavioural support. The primary outcome was exhaled carbon monoxide (CO)-verified continuous smoking abstinence 6 months after the agreed quit date. Primary analysis was by intention to treat, with sensitivity analysis by per protocol, treatment adherence, varying CO cutoffs, and complete case analysis. This paper presents the main analyses and is registered with ClinicalTrials.gov, NCT02521662.
Findings
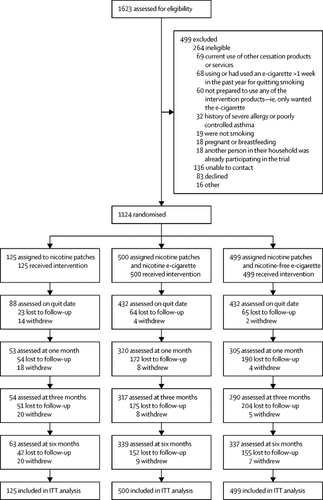
Between March 17, 2016 and Nov 30, 2017, 1124 people were assigned to nicotine patches (patches only group, n=125), patches plus a nicotine e-cigarette (patches plus nicotine e-cigarette group, n=500), or patches plus a nicotine-free e-cigarette (patches plus nicotine-free e-cigarette group, n=499). 62 (50%) of 125 participants in the patches only group withdrew or were lost to follow-up by 6 months compared with 161 (32%) of 500 in the patches plus nicotine e-cigarette group and 162 (33%) of 499 in the patches plus nicotine-free e-cigarette group. 35 (7%) participants in the patches plus nicotine e-cigarette group had CO-verified continuous abstinence at 6 months compared with 20 (4%) in the patches plus nicotine-free e-cigarette group (risk difference [RD] 2·99 [95% CI 0·17–5·81]), and three (2%) people in the patches only group (RD 4·60 [1·11–8·09]). 18 serious adverse events occurred in 16 people in the patches plus nicotine e-cigarette group compared with 27 events in 22 people in the patches plus nicotine-free e-cigarette group and four events in three people in the patches only group. In the patches plus nicotine e-cigarette group, two life-threatening serious adverse events were reported (two separate heart attacks in the one participant). In the patches plus nicotine-free e-cigarette group, one death occurred (accidental drug overdose) and one life-threatening serious adverse event (heart attack). No significant between-group differences were noted for serious adverse events, and none were treatment-related.
Interpretation
Combining reduced-harm nicotine products, such as nicotine patches with a nicotine e-cigarette, can lead to a modest improvement in smoking cessation over and above that obtained from using patches plus a nicotine-free e-cigarette (or patches alone), with no indication of any serious harm in the short-term. Future e-cigarette trials should focus on their use alone or in combination with usual smoking cessation support, given issues with differential loss to follow-up and withdrawal if a usual care group is used as a comparator.
Funding
Health Research Council of New Zealand.
中文翻译:

尼古丁贴剂与电子烟(有或没有尼古丁)结合使用以戒烟:一项实用的随机试验。
背景
联合尼古丁替代疗法显示出额外的戒烟益处。我们的目的是找出将尼古丁贴片与电子烟(有或没有尼古丁)相结合对戒烟六个月的有效性。
方法
我们在新西兰进行了一次务实的三臂平行小组试验,研究对象是那些天真的电子烟且积极戒烟的成年吸烟者。使用国家媒体广告从一般人群中招募参与者。使用分层分组随机法将参与者随机分配(1:4:4),以接受14周(同意退出日期的前2周)的21 mg,24h尼古丁贴剂,贴剂和18 mg / L尼古丁-香烟或贴剂,再加上不含尼古丁的电子烟。我们建议参与者每天使用一个贴片,并在必要或需要时使用电子烟。参与者和研究人员对电子烟碱的含量不了解。我们提供了为期6周的电话提供的行为支持。主要结局是在商定的戒烟日期后6个月呼出一氧化碳(CO)验证的连续戒烟。初步分析是根据治疗的意图进行的,根据方案的敏感性分析,治疗依从性,不同的CO临界值以及完整的病例分析。本文介绍了主要的分析方法,并已在ClinicalTrials.gov注册,NCT02521662。
发现
在2016年3月17日至2017年11月30日之间,有1124人被分配了尼古丁贴片(仅贴片组,n = 125),贴片和尼古丁电子烟(贴片和尼古丁电子烟组,n = 500),或补丁加上无尼古丁的电子烟(补丁加上无尼古丁的电子烟组,n = 499)。仅贴剂组的125名参与者中有62名(50%)退出或失去了随访6个月,而贴剂加尼古丁电子烟组的500名受试者中的161名(32%)和499名中的162名(33%)在贴片中加上不含尼古丁的电子烟。贴剂加尼古丁电子烟组中有35(7%)名参与者在6个月时进行了CO验证的连续禁欲,而贴剂加无尼古丁电子烟组中有20(4%)名患者进行了CO验证的连续禁欲(风险差异[RD] 2· 99 [95%CI 0·17–5·81]),仅补丁程序组中的三(2%)人(RD 4·60 [1·11-8·09])。贴剂加尼古丁电子烟组中有16人发生18严重不良事件,而贴剂加无尼古丁电子烟组中有22人中有27事件,仅贴剂组中有3人中有4事件。在贴剂加尼古丁电子烟组中,报告了两次危及生命的严重不良事件(一名参与者发生了两次独立的心脏病发作)。在贴剂加无尼古丁的电子烟组中,发生了1例死亡(意外用药过量)和1例危及生命的严重不良事件(心脏病发作)。没有发现严重不良事件的显着组间差异,也没有与治疗有关的差异。贴剂加尼古丁电子烟组中有16人发生18严重不良事件,而贴剂加无尼古丁电子烟组中有22人中有27事件,仅贴剂组中有3人中有4事件。在贴剂加尼古丁电子烟组中,报告了两次危及生命的严重不良事件(一名参与者发生了两次独立的心脏病发作)。在贴剂加无尼古丁的电子烟组中,发生了1例死亡(意外用药过量)和1例危及生命的严重不良事件(心脏病发作)。没有发现严重不良事件的显着组间差异,也没有与治疗有关的差异。贴剂加尼古丁电子烟组中有16人发生18严重不良事件,而贴剂加无尼古丁电子烟组中有22人中有27事件,仅贴剂组中有3人中有4事件。在贴剂加尼古丁电子烟组中,报告了两次危及生命的严重不良事件(一名参与者发生了两次独立的心脏病发作)。在贴剂加无尼古丁的电子烟组中,发生了1例死亡(意外用药过量)和1例危及生命的严重不良事件(心脏病发作)。没有发现严重不良事件的显着组间差异,也没有与治疗有关的差异。在贴剂加尼古丁电子烟组中,报告了两次危及生命的严重不良事件(一名参与者发生了两次独立的心脏病发作)。在贴剂加无尼古丁的电子烟组中,发生了1例死亡(意外用药过量)和1例危及生命的严重不良事件(心脏病发作)。没有发现严重不良事件的显着组间差异,也没有与治疗有关的差异。在贴剂加尼古丁电子烟组中,报告了两次危及生命的严重不良事件(一名参与者发生了两次独立的心脏病发作)。在贴剂加无尼古丁的电子烟组中,发生了1例死亡(意外用药过量)和1例危及生命的严重不良事件(心脏病发作)。没有发现严重不良事件的显着组间差异,也没有与治疗有关的差异。
解释
将危害较小的尼古丁产品(例如尼古丁贴片和尼古丁电子烟)相结合,可以使戒烟比使用贴片加无尼古丁的电子烟(或单独使用贴片)所获得的戒烟有所改善。短期内无任何严重危害的迹象。考虑到如果使用常规护理组作为比较者,在随访和戒断方面存在差异性损失的问题,未来的电子烟试验应侧重于单独使用或与常规戒烟支持结合使用。
资金
新西兰卫生研究委员会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号