Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cytosolic aggregates in presence of non-translocated proteins perturb endoplasmic reticulum structure and dynamics.
Traffic ( IF 3.6 ) Pub Date : 2019-10-01 , DOI: 10.1111/tra.12694 Debdatto Mookherjee 1 , Priyanka Majumder 1, 2 , Rukmini Mukherjee 1, 3 , Debmita Chatterjee 1 , Zenia Kaul 1, 4 , Subhrangshu Das 5 , Rachid Sougrat 6 , Saikat Chakrabarti 5 , Oishee Chakrabarti 1, 7
Traffic ( IF 3.6 ) Pub Date : 2019-10-01 , DOI: 10.1111/tra.12694 Debdatto Mookherjee 1 , Priyanka Majumder 1, 2 , Rukmini Mukherjee 1, 3 , Debmita Chatterjee 1 , Zenia Kaul 1, 4 , Subhrangshu Das 5 , Rachid Sougrat 6 , Saikat Chakrabarti 5 , Oishee Chakrabarti 1, 7
Affiliation
|
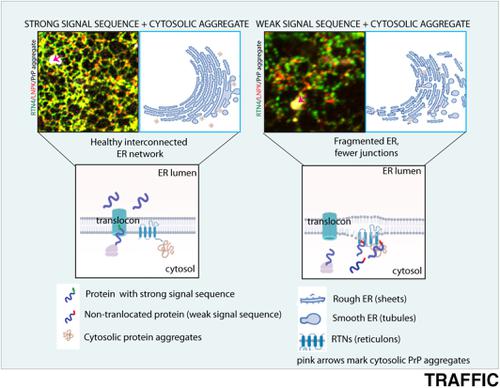
Presence of cytosolic protein aggregates and membrane damage are two common attributes of neurodegenerative diseases. These aggregates delay degradation of non-translocated protein precursors leading to their persistence and accumulation in the cytosol. Here, we find that cells with intracellular protein aggregates (of cytosolic prion protein or huntingtin) destabilize the endoplasmic reticulum (ER) morphology and dynamics when non-translocated protein load is high. This affects trafficking of proteins out from the ER, relative distribution of the rough and smooth ER and three-way junctions that are essential for the structural integrity of the membrane network. The changes in ER membranes may be due to high aggregation tendency of the ER structural proteins-reticulons, and altered distribution of those associated with the three-way ER junctions-Lunapark. Reticulon4 is seen to be enriched in the aggregate fractions in presence of non-translocated protein precursors. This could be mitigated by improving signal sequence efficiencies of the proteins targeted to the ER. These were observed using PrP variants and the seven-pass transmembrane protein (CRFR1) with different signal sequences that led to diverse translocation efficiencies. This identifies a previously unappreciated consequence of cytosolic aggregates on non-translocated precursor proteins-their persistent presence affects ER morphology and dynamics. This may be one of the ways in which cytosolic aggregates can affect endomembranes during neurodegenerative disease.
中文翻译:

存在非转运蛋白的胞质聚集体扰乱了内质网的结构和动力学。
胞浆蛋白聚集体的存在和膜损伤是神经退行性疾病的两个常见属性。这些聚集体延迟了非易位蛋白前体的降解,从而导致其在胞质溶胶中的持久性和积累。在这里,我们发现当非易位蛋白负荷很高时,具有胞内蛋白聚集体(胞质病毒蛋白或亨廷顿蛋白)的细胞会破坏内质网(ER)的形态和动力学。这会影响蛋白质从内质网中运出,粗糙和光滑内质网的相对分布以及三向连接,这对于膜网络的结构完整性至关重要。ER膜的变化可能是由于ER结构蛋白网状蛋白的高聚集趋势所致,并且改变了与三通ER交叉口-卢纳帕克(Lunapark)相关的分布。在非易位蛋白前体的存在下,Reticulon4富含聚集部分。这可以通过改善靶向ER的蛋白质的信号序列效率来减轻。使用PrP变体和具有不同信号序列的七遍跨膜蛋白(CRFR1)观察到了这些结果,从而导致了多种转运效率。这确定了胞浆聚集体对未转运的前体蛋白的先前未曾认识到的结果-它们的持续存在会影响ER的形态和动力学。这可能是神经退行性疾病期间胞质聚集体影响内膜的方式之一。在非易位蛋白前体的存在下,Reticulon4富含聚集部分。这可以通过改善靶向ER的蛋白质的信号序列效率来减轻。使用PrP变体和具有不同信号序列的七遍跨膜蛋白(CRFR1)观察到了这些结果,从而导致了多种转运效率。这确定了胞浆聚集体对未转运的前体蛋白的先前未曾认识到的结果-它们的持续存在会影响ER的形态和动力学。这可能是神经退行性疾病期间胞质聚集体影响内膜的方式之一。在非易位蛋白前体的存在下,Reticulon4富含聚集部分。这可以通过改善靶向ER的蛋白质的信号序列效率来减轻。使用PrP变体和具有不同信号序列的七遍跨膜蛋白(CRFR1)观察到了这些结果,从而导致了多种转运效率。这确定了胞浆聚集体对未转运的前体蛋白的先前未曾认识到的结果-它们的持续存在会影响ER的形态和动力学。这可能是神经退行性疾病期间胞质聚集体影响内膜的方式之一。使用PrP变体和具有不同信号序列的七遍跨膜蛋白(CRFR1)观察到了这些结果,从而导致了多种转运效率。这确定了胞浆聚集体对未转运的前体蛋白的先前未曾认识到的结果-它们的持续存在会影响ER的形态和动力学。这可能是神经退行性疾病期间胞质聚集体影响内膜的方式之一。使用PrP变体和具有不同信号序列的七遍跨膜蛋白(CRFR1)观察到了这些结果,从而导致了多种转运效率。这确定了胞浆聚集体对未转运的前体蛋白的先前未曾认识到的结果-它们的持续存在会影响ER的形态和动力学。这可能是神经退行性疾病期间胞质聚集体影响内膜的方式之一。
更新日期:2019-10-01
中文翻译:

存在非转运蛋白的胞质聚集体扰乱了内质网的结构和动力学。
胞浆蛋白聚集体的存在和膜损伤是神经退行性疾病的两个常见属性。这些聚集体延迟了非易位蛋白前体的降解,从而导致其在胞质溶胶中的持久性和积累。在这里,我们发现当非易位蛋白负荷很高时,具有胞内蛋白聚集体(胞质病毒蛋白或亨廷顿蛋白)的细胞会破坏内质网(ER)的形态和动力学。这会影响蛋白质从内质网中运出,粗糙和光滑内质网的相对分布以及三向连接,这对于膜网络的结构完整性至关重要。ER膜的变化可能是由于ER结构蛋白网状蛋白的高聚集趋势所致,并且改变了与三通ER交叉口-卢纳帕克(Lunapark)相关的分布。在非易位蛋白前体的存在下,Reticulon4富含聚集部分。这可以通过改善靶向ER的蛋白质的信号序列效率来减轻。使用PrP变体和具有不同信号序列的七遍跨膜蛋白(CRFR1)观察到了这些结果,从而导致了多种转运效率。这确定了胞浆聚集体对未转运的前体蛋白的先前未曾认识到的结果-它们的持续存在会影响ER的形态和动力学。这可能是神经退行性疾病期间胞质聚集体影响内膜的方式之一。在非易位蛋白前体的存在下,Reticulon4富含聚集部分。这可以通过改善靶向ER的蛋白质的信号序列效率来减轻。使用PrP变体和具有不同信号序列的七遍跨膜蛋白(CRFR1)观察到了这些结果,从而导致了多种转运效率。这确定了胞浆聚集体对未转运的前体蛋白的先前未曾认识到的结果-它们的持续存在会影响ER的形态和动力学。这可能是神经退行性疾病期间胞质聚集体影响内膜的方式之一。在非易位蛋白前体的存在下,Reticulon4富含聚集部分。这可以通过改善靶向ER的蛋白质的信号序列效率来减轻。使用PrP变体和具有不同信号序列的七遍跨膜蛋白(CRFR1)观察到了这些结果,从而导致了多种转运效率。这确定了胞浆聚集体对未转运的前体蛋白的先前未曾认识到的结果-它们的持续存在会影响ER的形态和动力学。这可能是神经退行性疾病期间胞质聚集体影响内膜的方式之一。使用PrP变体和具有不同信号序列的七遍跨膜蛋白(CRFR1)观察到了这些结果,从而导致了多种转运效率。这确定了胞浆聚集体对未转运的前体蛋白的先前未曾认识到的结果-它们的持续存在会影响ER的形态和动力学。这可能是神经退行性疾病期间胞质聚集体影响内膜的方式之一。使用PrP变体和具有不同信号序列的七遍跨膜蛋白(CRFR1)观察到了这些结果,从而导致了多种转运效率。这确定了胞浆聚集体对未转运的前体蛋白的先前未曾认识到的结果-它们的持续存在会影响ER的形态和动力学。这可能是神经退行性疾病期间胞质聚集体影响内膜的方式之一。











































 京公网安备 11010802027423号
京公网安备 11010802027423号