当前位置:
X-MOL 学术
›
Adv. Sust. Syst.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water‐Soluble Organic Dyes as Molecular Photocatalysts for H2O2 Evolution
Advanced Sustainable Systems ( IF 6.5 ) Pub Date : 2019-05-13 , DOI: 10.1002/adsu.201900027 Maciej Gryszel 1, 2 , Renata Rybakiewicz 3, 4 , Eric Daniel Głowacki 1, 2
Advanced Sustainable Systems ( IF 6.5 ) Pub Date : 2019-05-13 , DOI: 10.1002/adsu.201900027 Maciej Gryszel 1, 2 , Renata Rybakiewicz 3, 4 , Eric Daniel Głowacki 1, 2
Affiliation
|
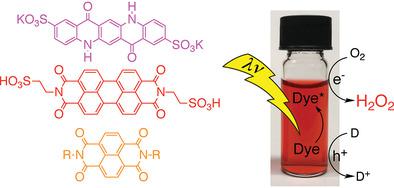
Photochemical generation of hydrogen peroxide via oxygen reduction is a critical component of emerging sustainable energy conversion concepts. Light‐absorbing semiconductors as well as electrodes modified with sensitizers typically catalyze oxygen photoreduction to hydrogen peroxide. Here, it is reported that, in contrast to these heterogeneous systems, a homogeneous solution of a metal‐free organic dye can perform the whole catalytic cycle of hydrogen peroxide photoevolution itself. This cycle can proceed with simultaneous oxidation of various organic molecules as electron donors, or even water. In the three water‐soluble dyes that are experimented with, photoevolution of peroxide occurs favorably at neutral to basic pH. The reaction is first order with respect to dye concentration, and evidence implicates a single‐electron reduction pathway with superoxide as an intermediate. Photostability of the dyes over time correlates with increased oxidation potential of the molecule. The finding that hydrogen peroxide can be produced in aqueous solution with single fully organic molecules performing the entire photocatalytic cycle creates a new avenue for the peroxide carbon free energy cycle.
中文翻译:

水溶性有机染料分子作为光催化剂用于h 2 Ø 2进化
通过减少氧气以光化学方式产生过氧化氢是新兴的可持续能源转换概念的关键组成部分。吸光半导体以及用敏化剂修饰的电极通常会催化氧气光还原为过氧化氢。在这里,据报道,与这些异质体系相比,无金属有机染料的均质溶液可以执行过氧化氢自身光催化演化的整个催化循环。该循环可以同时氧化各种有机分子作为电子给体,甚至是水。在所试验的三种水溶性染料中,过氧化物在中性至碱性pH值下会发生光进化。该反应是关于染料浓度的一级反应,有证据表明,以超氧化物为中间体的单电子还原途径。染料随时间的光稳定性与分子氧化电位的增加有关。过氧化氢可以在单一的完全有机分子的水溶液中完成整个光催化循环的过程中产生,这一发现为过氧化物无碳能量循环创造了一条新途径。
更新日期:2019-09-09
中文翻译:

水溶性有机染料分子作为光催化剂用于h 2 Ø 2进化
通过减少氧气以光化学方式产生过氧化氢是新兴的可持续能源转换概念的关键组成部分。吸光半导体以及用敏化剂修饰的电极通常会催化氧气光还原为过氧化氢。在这里,据报道,与这些异质体系相比,无金属有机染料的均质溶液可以执行过氧化氢自身光催化演化的整个催化循环。该循环可以同时氧化各种有机分子作为电子给体,甚至是水。在所试验的三种水溶性染料中,过氧化物在中性至碱性pH值下会发生光进化。该反应是关于染料浓度的一级反应,有证据表明,以超氧化物为中间体的单电子还原途径。染料随时间的光稳定性与分子氧化电位的增加有关。过氧化氢可以在单一的完全有机分子的水溶液中完成整个光催化循环的过程中产生,这一发现为过氧化物无碳能量循环创造了一条新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号