Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial.
The Lancet ( IF 98.4 ) Pub Date : 2019-09-05 , DOI: 10.1016/s0140-6736(19)31817-3 Bruce A C Cree 1 , Jeffrey L Bennett 2 , Ho Jin Kim 3 , Brian G Weinshenker 4 , Sean J Pittock 4 , Dean M Wingerchuk 5 , Kazuo Fujihara 6 , Friedemann Paul 7 , Gary R Cutter 8 , Romain Marignier 9 , Ari J Green 10 , Orhan Aktas 11 , Hans-Peter Hartung 11 , Fred D Lublin 12 , Jorn Drappa 13 , Gerard Barron 14 , Soraya Madani 13 , John N Ratchford 13 , Dewei She 13 , Daniel Cimbora 13 , Eliezer Katz 13 ,
The Lancet ( IF 98.4 ) Pub Date : 2019-09-05 , DOI: 10.1016/s0140-6736(19)31817-3 Bruce A C Cree 1 , Jeffrey L Bennett 2 , Ho Jin Kim 3 , Brian G Weinshenker 4 , Sean J Pittock 4 , Dean M Wingerchuk 5 , Kazuo Fujihara 6 , Friedemann Paul 7 , Gary R Cutter 8 , Romain Marignier 9 , Ari J Green 10 , Orhan Aktas 11 , Hans-Peter Hartung 11 , Fred D Lublin 12 , Jorn Drappa 13 , Gerard Barron 14 , Soraya Madani 13 , John N Ratchford 13 , Dewei She 13 , Daniel Cimbora 13 , Eliezer Katz 13 ,
Affiliation
|
BACKGROUND
No approved therapies exist for neuromyelitis optica spectrum disorder (NMOSD), a rare, relapsing, autoimmune, inflammatory disease of the CNS that causes blindness and paralysis. We aimed to assess the efficacy and safety of inebilizumab, an anti-CD19, B cell-depleting antibody, in reducing the risk of attacks and disability in NMOSD.
METHODS
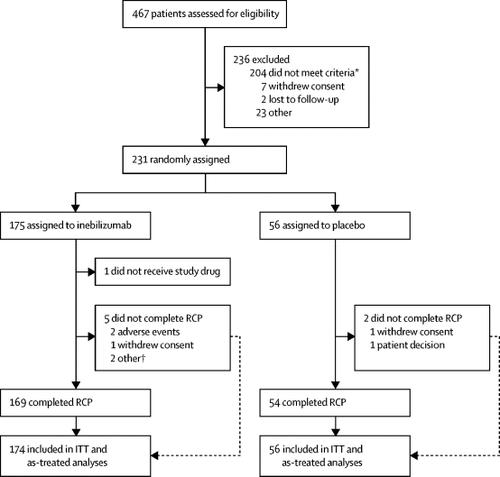
We did a multicentre, double-blind, randomised placebo-controlled phase 2/3 study at 99 outpatient specialty clinics or hospitals in 25 countries. Eligible participants were adults (≥18 years old) with a diagnosis of NMOSD, an Expanded Disability Status Scale score of 8·0 or less, and a history of at least one attack requiring rescue therapy in the year before screening or at least two attacks requiring rescue therapy in the 2 years before screening. Participants were randomly allocated (3:1) to 300 mg intravenous inebilizumab or placebo with a central interactive voice response system or interactive web response system and permuted block randomisation. Inebilizumab or placebo was administered on days 1 and 15. Participants, investigators, and all clinical staff were masked to the treatments, and inebilizumab and placebo were indistinguishable in appearance. The primary endpoint was time to onset of an NMOSD attack, as determined by the adjudication committee. Efficacy endpoints were assessed in all randomly allocated patients who received at least one dose of study intervention, and safety endpoints were assessed in the as-treated population. The study is registered with ClinicalTrials.gov, number NCT02200770.
FINDINGS
Between Jan 6, 2015, and Sept 24, 2018, 230 participants were randomly assigned to treatment and dosed, with 174 participants receiving inebilizumab and 56 receiving placebo. The randomised controlled period was stopped before complete enrolment, as recommended by the independent data-monitoring committee, because of a clear demonstration of efficacy. 21 (12%) of 174 participants receiving inebilizumab had an attack versus 22 (39%) of 56 participants receiving placebo (hazard ratio 0·272 [95% CI 0·150-0·496]; p<0·0001). Adverse events occurred in 125 (72%) of 174 participants receiving inebilizumab and 41 (73%) of 56 participants receiving placebo. Serious adverse events occurred in eight (5%) of 174 participants receiving inebilizumab and five (9%) of 56 participants receiving placebo.
INTERPRETATION
Compared with placebo, inebilizumab reduced the risk of an NMOSD attack. Inebilizumab has potential application as an evidence-based treatment for patients with NMOSD.
FUNDING
MedImmune and Viela Bio.
中文翻译:

依那利珠单抗用于治疗视神经脊髓炎频谱疾病(N-动量):一项双盲,随机安慰剂对照的2/3期试验。
背景技术不存在用于视神经脊髓炎光谱疾病(NMOSD)的批准的疗法,该疾病是引起失明和麻痹的CNS的罕见,复发性,自身免疫,炎性疾病。我们旨在评估抗CD19,B细胞消耗性抗体inebilizumab在降低NMOSD发作和致残风险方面的功效和安全性。方法我们在25个国家/地区的99个门诊专科诊所或医院进行了多中心,双盲,随机安慰剂对照的2/3期研究。符合条件的参与者为诊断为NMOSD的成人(≥18岁),扩展的残疾状态量表得分为8·0或更低,并且在筛查前一年中至少有一次需要抢救治疗的发作或至少两次发作的病史在筛查之前的2年内需要抢救治疗。参与者是随机分配的(3:1)300毫克静脉注射inebilizumab或安慰剂,并带有中央交互式语音应答系统或交互式网络应答系统,并进行区组随机分组。在第1天和第15天使用了Inebilizumab或安慰剂。参与者,研究者和所有临床人员均被屏蔽了治疗,并且inebilizumab和安慰剂的外观无明显区别。根据审判委员会的决定,主要终点是爆发NMOSD发作的时间。在接受至少一剂研究干预的所有随机分配的患者中评估疗效终点,并在接受治疗的人群中评估安全终点。该研究已在ClinicalTrials.gov上注册,编号为NCT02200770。研究结果在2015年1月6日至2018年9月24日之间,随机分配了230名参与者进行治疗,174名参与者接受了inebilizumab,56名参与者接受了安慰剂。根据独立数据监测委员会的建议,由于完全可以证明疗效,因此在完全入组之前就停止了随机对照期。接受inebilizumab的174名参与者中有21名(12%)发作,而接受安慰剂的56名参与者中有22(39%)名发生发作(危险比0·272 [95%CI 0·150-0·496]; p <0·0001)。接受inebilizumab的174位参与者中有125位(72%)发生不良事件,接受安慰剂的56位参与者中41位(73%)发生了不良事件。接受inebilizumab的174名参与者中有八名(5%)发生严重不良事件,接受安慰剂的56名参与者中有五名(9%)发生。解释与安慰剂相比,伊尼珠单抗降低了NMOSD发作的风险。依那利珠单抗作为NMOSD患者的循证疗法具有潜在的应用前景。资助MedImmune和Viela Bio。
更新日期:2019-10-12
中文翻译:

依那利珠单抗用于治疗视神经脊髓炎频谱疾病(N-动量):一项双盲,随机安慰剂对照的2/3期试验。
背景技术不存在用于视神经脊髓炎光谱疾病(NMOSD)的批准的疗法,该疾病是引起失明和麻痹的CNS的罕见,复发性,自身免疫,炎性疾病。我们旨在评估抗CD19,B细胞消耗性抗体inebilizumab在降低NMOSD发作和致残风险方面的功效和安全性。方法我们在25个国家/地区的99个门诊专科诊所或医院进行了多中心,双盲,随机安慰剂对照的2/3期研究。符合条件的参与者为诊断为NMOSD的成人(≥18岁),扩展的残疾状态量表得分为8·0或更低,并且在筛查前一年中至少有一次需要抢救治疗的发作或至少两次发作的病史在筛查之前的2年内需要抢救治疗。参与者是随机分配的(3:1)300毫克静脉注射inebilizumab或安慰剂,并带有中央交互式语音应答系统或交互式网络应答系统,并进行区组随机分组。在第1天和第15天使用了Inebilizumab或安慰剂。参与者,研究者和所有临床人员均被屏蔽了治疗,并且inebilizumab和安慰剂的外观无明显区别。根据审判委员会的决定,主要终点是爆发NMOSD发作的时间。在接受至少一剂研究干预的所有随机分配的患者中评估疗效终点,并在接受治疗的人群中评估安全终点。该研究已在ClinicalTrials.gov上注册,编号为NCT02200770。研究结果在2015年1月6日至2018年9月24日之间,随机分配了230名参与者进行治疗,174名参与者接受了inebilizumab,56名参与者接受了安慰剂。根据独立数据监测委员会的建议,由于完全可以证明疗效,因此在完全入组之前就停止了随机对照期。接受inebilizumab的174名参与者中有21名(12%)发作,而接受安慰剂的56名参与者中有22(39%)名发生发作(危险比0·272 [95%CI 0·150-0·496]; p <0·0001)。接受inebilizumab的174位参与者中有125位(72%)发生不良事件,接受安慰剂的56位参与者中41位(73%)发生了不良事件。接受inebilizumab的174名参与者中有八名(5%)发生严重不良事件,接受安慰剂的56名参与者中有五名(9%)发生。解释与安慰剂相比,伊尼珠单抗降低了NMOSD发作的风险。依那利珠单抗作为NMOSD患者的循证疗法具有潜在的应用前景。资助MedImmune和Viela Bio。











































 京公网安备 11010802027423号
京公网安备 11010802027423号