Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Low-Intensity vs Standard-Intensity Warfarin Prophylaxis on Venous Thromboembolism or Death Among Patients Undergoing Hip or Knee Arthroplasty
JAMA ( IF 63.1 ) Pub Date : 2019-09-03 , DOI: 10.1001/jama.2019.12085 Brian F Gage 1 , Anne R Bass 2 , Hannah Lin 1, 3 , Scott C Woller 4, 5 , Scott M Stevens 4, 5 , Noor Al-Hammadi 1 , Jeffrey L Anderson 5, 6 , Juan Li 1 , Tomás Rodriguez 1 , J Philip Miller 1 , Gwendolyn A McMillin 7 , Robert C Pendleton 5 , Amir K Jaffer 8 , Cristi R King 1 , Brandi Whipple 1 , Rhonda Porche-Sorbet 1 , Lynnae Napoli 5 , Kerri Merritt 2 , Anna M Thompson 1, 9 , Gina Hyun 1, 10 , Wesley Hollomon 11 , Robert L Barrack 12 , Ryan M Nunley 12 , Gerard Moskowitz 1 , Victor Dávila-Román 1 , Charles S Eby 1, 13
JAMA ( IF 63.1 ) Pub Date : 2019-09-03 , DOI: 10.1001/jama.2019.12085 Brian F Gage 1 , Anne R Bass 2 , Hannah Lin 1, 3 , Scott C Woller 4, 5 , Scott M Stevens 4, 5 , Noor Al-Hammadi 1 , Jeffrey L Anderson 5, 6 , Juan Li 1 , Tomás Rodriguez 1 , J Philip Miller 1 , Gwendolyn A McMillin 7 , Robert C Pendleton 5 , Amir K Jaffer 8 , Cristi R King 1 , Brandi Whipple 1 , Rhonda Porche-Sorbet 1 , Lynnae Napoli 5 , Kerri Merritt 2 , Anna M Thompson 1, 9 , Gina Hyun 1, 10 , Wesley Hollomon 11 , Robert L Barrack 12 , Ryan M Nunley 12 , Gerard Moskowitz 1 , Victor Dávila-Román 1 , Charles S Eby 1, 13
Affiliation
|
Importance
The optimal international normalized ratio (INR) to prevent venous thromboembolism (VTE) in warfarin-treated patients with recent arthroplasty is unknown. Objective
To determine the safety and efficacy of a target INR of 1.8 vs 2.5 for VTE prophylaxis after orthopedic surgery. Design, Setting, and Participants
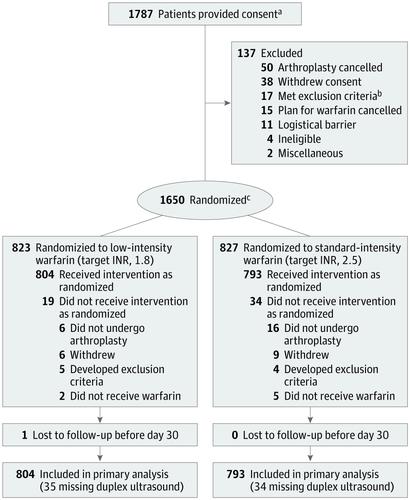
The randomized Genetic Informatics Trial (GIFT) of Warfarin to Prevent Deep Vein Thrombosis enrolled 1650 patients aged 65 years or older initiating warfarin for elective hip or knee arthroplasty at 6 US medical centers. Enrollment began in April 2011 and follow-up concluded in October 2016. Interventions
In a 2 × 2 factorial design, participants were randomized to a target INR of 1.8 (n = 823) or 2.5 (n = 827) and to either genotype-guided or clinically guided warfarin dosing. For the first 11 days of therapy, open-label warfarin dosing was guided by a web application. Main Outcomes and Measures
The primary outcome was the composite of VTE (within 60 days) or death (within 30 days). Participants underwent screening duplex ultrasound postoperatively. The hypothesis was that an INR target of 1.8 would be noninferior to an INR target of 2.5, using a noninferiority margin of 3% for the absolute risk of VTE. Secondary end points were bleeding and INR values of 4 or more. Results
Among 1650 patients who were randomized (mean age, 72.1 years; 1049 women [63.6%]; 1502 white [91.0%]), 1597 (96.8%) received at least 1 dose of warfarin and were included in the primary analysis. The rate of the primary composite outcome of VTE or death was 5.1% (41 of 804) in the low-intensity-warfarin group (INR target, 1.8) vs 3.8% (30 of 793) in the standard-treatment-warfarin group (INR target, 2.5), for a difference of 1.3% (1-sided 95% CI, -∞ to 3.05%, P = .06 for noninferiority). Major bleeding occurred in 0.4% of patients in the low-intensity group and 0.9% of patients in the standard-intensity group, for a difference of -0.5% (95% CI, -1.6% to 0.4%). The INR values of 4 or more occurred in 4.5% of patients in the low-intensity group and 12.2% of the standard-intensity group, for a difference of -7.8% (95% CI, -10.5% to -5.1%). Conclusions and Relevance
Among older patients undergoing hip or knee arthroplasty and receiving warfarin prophylaxis, an international normalized ratio goal of 1.8 compared with 2.5 did not meet the criterion for noninferiority for risk of the composite outcome of VTE or death. However, the trial may have been underpowered to meet this criterion and further research may be warranted. Trial Registration
ClinicalTrials.gov Identifier: NCT01006733.
中文翻译:

低强度与标准强度华法林预防对髋关节或膝关节置换术患者静脉血栓栓塞或死亡的影响
重要性 对于近期接受过关节置换术的华法林治疗患者预防静脉血栓栓塞 (VTE) 的最佳国际标准化比率 (INR) 尚不清楚。目的 确定 1.8 和 2.5 的目标 INR 在骨科手术后预防 VTE 的安全性和有效性。设计、设置和参与者 华法林预防深静脉血栓形成的随机遗传信息学试验 (GIFT) 在 6 个美国医疗中心招募了 1650 名 65 岁或以上开始华法林进行选择性髋关节或膝关节置换术的患者。招募于 2011 年 4 月开始,随访于 2016 年 10 月结束。 干预 在 2 × 2 因子设计中,参与者被随机分配到目标 INR 为 1.8 (n = 823) 或 2.5 (n = 827) 和基因型指导或临床指导的华法林剂量。在治疗的前 11 天,开放标签华法林剂量由网络应用程序指导。主要结果和措施 主要结果是 VTE(60 天内)或死亡(30 天内)的复合。参与者在术后接受了双工超声筛查。假设是 1.8 的 INR 目标不劣于 2.5 的 INR 目标,对 VTE 的绝对风险使用 3% 的非劣效边际。次要终点是出血和 INR 值为 4 或更高。结果 在随机分组的 1650 名患者中(平均年龄 72.1 岁;1049 名女性 [63.6%];1502 名白人 [91.0%]),1597 名(96.8%)至少接受了 1 剂华法林并被纳入主要分析。低强度华法林组(INR 目标,1.8)vs 3 的 VTE 或死亡的主要复合结局发生率为 5.1%(804 人中的 41 人)。标准治疗华法林组为 8%(793 人中的 30 人)(INR 目标,2.5),差异为 1.3%(单侧 95% CI,-∞ 至 3.05%,非劣效性 P = .06)。低强度组 0.4% 的患者和标准强度组 0.9% 的患者发生大出血,差异为 -0.5%(95% CI,-1.6% 至 0.4%)。4.5% 的低强度组患者和 12.2% 的标准强度组患者的 INR 值为 4 或更高,差异为 -7.8%(95% CI,-10.5% 至 -5.1%)。结论和相关性 在接受髋关节或膝关节置换术并接受华法林预防的老年患者中,1.8 与 2.5 的国际标准化比率目标不符合 VTE 或死亡复合结局风险的非劣效性标准。然而,该试验可能不足以满足这一标准,可能需要进一步研究。试验注册 ClinicalTrials.gov 标识符:NCT01006733。
更新日期:2019-09-03
中文翻译:

低强度与标准强度华法林预防对髋关节或膝关节置换术患者静脉血栓栓塞或死亡的影响
重要性 对于近期接受过关节置换术的华法林治疗患者预防静脉血栓栓塞 (VTE) 的最佳国际标准化比率 (INR) 尚不清楚。目的 确定 1.8 和 2.5 的目标 INR 在骨科手术后预防 VTE 的安全性和有效性。设计、设置和参与者 华法林预防深静脉血栓形成的随机遗传信息学试验 (GIFT) 在 6 个美国医疗中心招募了 1650 名 65 岁或以上开始华法林进行选择性髋关节或膝关节置换术的患者。招募于 2011 年 4 月开始,随访于 2016 年 10 月结束。 干预 在 2 × 2 因子设计中,参与者被随机分配到目标 INR 为 1.8 (n = 823) 或 2.5 (n = 827) 和基因型指导或临床指导的华法林剂量。在治疗的前 11 天,开放标签华法林剂量由网络应用程序指导。主要结果和措施 主要结果是 VTE(60 天内)或死亡(30 天内)的复合。参与者在术后接受了双工超声筛查。假设是 1.8 的 INR 目标不劣于 2.5 的 INR 目标,对 VTE 的绝对风险使用 3% 的非劣效边际。次要终点是出血和 INR 值为 4 或更高。结果 在随机分组的 1650 名患者中(平均年龄 72.1 岁;1049 名女性 [63.6%];1502 名白人 [91.0%]),1597 名(96.8%)至少接受了 1 剂华法林并被纳入主要分析。低强度华法林组(INR 目标,1.8)vs 3 的 VTE 或死亡的主要复合结局发生率为 5.1%(804 人中的 41 人)。标准治疗华法林组为 8%(793 人中的 30 人)(INR 目标,2.5),差异为 1.3%(单侧 95% CI,-∞ 至 3.05%,非劣效性 P = .06)。低强度组 0.4% 的患者和标准强度组 0.9% 的患者发生大出血,差异为 -0.5%(95% CI,-1.6% 至 0.4%)。4.5% 的低强度组患者和 12.2% 的标准强度组患者的 INR 值为 4 或更高,差异为 -7.8%(95% CI,-10.5% 至 -5.1%)。结论和相关性 在接受髋关节或膝关节置换术并接受华法林预防的老年患者中,1.8 与 2.5 的国际标准化比率目标不符合 VTE 或死亡复合结局风险的非劣效性标准。然而,该试验可能不足以满足这一标准,可能需要进一步研究。试验注册 ClinicalTrials.gov 标识符:NCT01006733。











































 京公网安备 11010802027423号
京公网安备 11010802027423号