当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of the Migraine Prevention Therapeutic Aimovig (Erenumab), the First FDA-Approved Antibody against a G-Protein-Coupled Receptor.
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2019-09-03 , DOI: 10.1021/acsptsci.9b00061 Chadwick Terence King 1 , Colin V Gegg 1 , Sylvia Nai-Yu Hu 1 , Hsieng Sen Lu 1 , Brian M Chan 1 , Kelly A Berry 1 , David W Brankow 1 , Tom J Boone 1 , Nebojsa Kezunovic 1 , Matt R Kelley 1 , Licheng Shi 1 , Cen Xu 1
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2019-09-03 , DOI: 10.1021/acsptsci.9b00061 Chadwick Terence King 1 , Colin V Gegg 1 , Sylvia Nai-Yu Hu 1 , Hsieng Sen Lu 1 , Brian M Chan 1 , Kelly A Berry 1 , David W Brankow 1 , Tom J Boone 1 , Nebojsa Kezunovic 1 , Matt R Kelley 1 , Licheng Shi 1 , Cen Xu 1
Affiliation
|
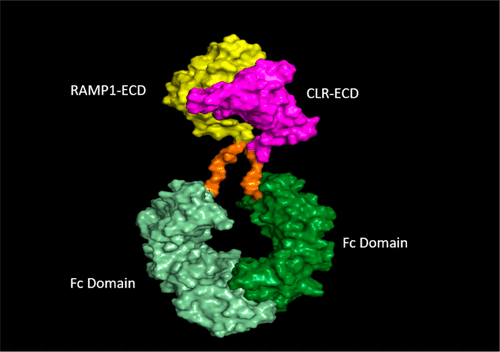
In 2018, the United States Food and Drug Administration (FDA) approved Aimovig (erenumab) for the prevention of migraine. Erenumab is the first FDA approved antibody therapeutic against a G-protein-coupled receptor, the canonical receptor of calcitonin gene related peptide (CGRP-R). A novel, epitope-focused antigen was created to reconstruct the extracellular domains of the CGRP-R in a stable conformation. Successful inoculation of XenoMouse animals and careful screening yielded multiple candidate molecules for high potency and exquisite selectivity toward the CGRP-R over related receptors. These efforts led to the discovery of erenumab which has demonstrated the desired efficacy and safety profiles in multiple clinical studies for the prevention of migraine. The innovation developed in the discovery of erenumab furthers the ability to target G-coupled protein receptors using antibody approaches.
中文翻译:

发现偏头痛预防治疗药Aimovig(Erenumab),这是首个获得FDA批准的抗G蛋白偶联受体的抗体。
2018年,美国食品药品监督管理局(FDA)批准了Aimovig(erenumab)用于预防偏头痛。Erenumab是首个获得FDA批准的抗G蛋白偶联受体(降钙素基因相关肽的规范受体(CGRP-R))的抗体。创建了一种新颖的,针对抗原决定簇的抗原,以稳定的构象重建了CGRP-R的胞外域。对XenoMouse动物的成功接种和仔细的筛选产生了多个候选分子,它们对CGRP-R的敏感性和对相关受体的选择性都很高。这些努力导致了埃仑单抗的发现,该埃仑单抗已在预防偏头痛的多项临床研究中证明了所需的功效和安全性。
更新日期:2019-09-03
中文翻译:

发现偏头痛预防治疗药Aimovig(Erenumab),这是首个获得FDA批准的抗G蛋白偶联受体的抗体。
2018年,美国食品药品监督管理局(FDA)批准了Aimovig(erenumab)用于预防偏头痛。Erenumab是首个获得FDA批准的抗G蛋白偶联受体(降钙素基因相关肽的规范受体(CGRP-R))的抗体。创建了一种新颖的,针对抗原决定簇的抗原,以稳定的构象重建了CGRP-R的胞外域。对XenoMouse动物的成功接种和仔细的筛选产生了多个候选分子,它们对CGRP-R的敏感性和对相关受体的选择性都很高。这些努力导致了埃仑单抗的发现,该埃仑单抗已在预防偏头痛的多项临床研究中证明了所需的功效和安全性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号