Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial.
The Lancet ( IF 98.4 ) Pub Date : 2019-09-03 , DOI: 10.1016/s0140-6736(19)31872-0 Pascal Vranckx 1 , Marco Valgimigli 2 , Lars Eckardt 3 , Jan Tijssen 4 , Thorsten Lewalter 5 , Giuseppe Gargiulo 6 , Valerii Batushkin 7 , Gianluca Campo 8 , Zoreslava Lysak 9 , Igor Vakaliuk 10 , Krzysztof Milewski 11 , Petra Laeis 12 , Paul-Egbert Reimitz 12 , Rüdiger Smolnik 12 , Wolfgang Zierhut 12 , Andreas Goette 13
The Lancet ( IF 98.4 ) Pub Date : 2019-09-03 , DOI: 10.1016/s0140-6736(19)31872-0 Pascal Vranckx 1 , Marco Valgimigli 2 , Lars Eckardt 3 , Jan Tijssen 4 , Thorsten Lewalter 5 , Giuseppe Gargiulo 6 , Valerii Batushkin 7 , Gianluca Campo 8 , Zoreslava Lysak 9 , Igor Vakaliuk 10 , Krzysztof Milewski 11 , Petra Laeis 12 , Paul-Egbert Reimitz 12 , Rüdiger Smolnik 12 , Wolfgang Zierhut 12 , Andreas Goette 13
Affiliation
|
BACKGROUND
We aimed to assess the safety of edoxaban in combination with P2Y12 inhibition in patients with atrial fibrillation who had percutaneous coronary intervention (PCI).
METHODS
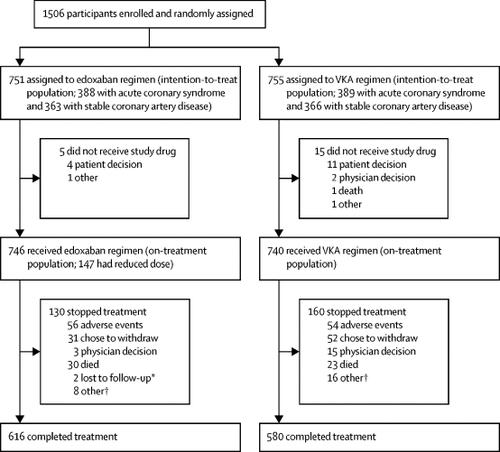
ENTRUST-AF PCI was a randomised, multicentre, open-label, non-inferiority phase 3b trial with masked outcome evaluation, done at 186 sites in 18 countries. Patients had atrial fibrillation requiring oral anticoagulation, were aged at least 18 years, and had a successful PCI for stable coronary artery disease or acute coronary syndrome. Participants were randomly assigned (1:1) from 4 h to 5 days after PCI using concealed, stratified, and blocked web-based central randomisation to either edoxaban (60 mg once daily) plus a P2Y12 inhibitor for 12 months or a vitamin K antagonist (VKA) in combination with a P2Y12 inhibitor and aspirin (100 mg once daily, for 1-12 months). The edoxaban dose was reduced to 30 mg per day if one or more factors (creatinine clearance 15-50 mL/min, bodyweight ≤60 kg, or concomitant use of specified potent P-glycoprotein inhibitors) were present. The primary endpoint was a composite of major or clinically relevant non-major (CRNM) bleeding within 12 months. The primary analysis was done in the intention-to-treat population and safety was assessed in all patients who received at least one dose of their assigned study drug. This trial is registered with ClinicalTrials.gov, NCT02866175, is closed to new participants, and follow-up is completed.
FINDINGS
From Feb 24, 2017, through May 7, 2018, 1506 patients were enrolled and randomly assigned to the edoxaban regimen (n=751) or VKA regimen (n=755). Median time from PCI to randomisation was 45·1 h (IQR 22·2-76·2). Major or CRNM bleeding events occurred in 128 (17%) of 751 patients (annualised event rate 20·7%) with the edoxaban regimen and 152 (20%) of 755 patients (annualised event rate 25·6%) patients with the VKA regimen; hazard ratio 0·83 (95% CI 0·65-1·05; p=0·0010 for non-inferiority, margin hazard ratio 1·20; p=0·1154 for superiority).
INTERPRETATION
In patients with atrial fibrillation who had PCI, the edoxaban-based regimen was non-inferior for bleeding compared with the VKA-based regimen, without significant differences in ischaemic events.
FUNDING
Daiichi Sankyo.
中文翻译:

在房颤患者成功完成冠状动脉支架置入术后,使用基于依多沙班和基于维生素K拮抗剂的抗血栓治疗方案(ENTRUST-AF PCI):一项随机,开放标签的3b期试验。
背景我们旨在评估依多沙班联合P2Y12抑制在经皮冠状动脉介入治疗(PCI)的房颤患者中的安全性。方法ENTRUST-AF PCI是一项随机,多中心,开放性,非劣效性3b期临床试验,其掩盖的结果评估在18个国家的186个地点进行。患有房颤的患者需要口服抗凝药物,年龄至少18岁,并且成功获得了可用于稳定冠状动脉疾病或急性冠状动脉综合征的PCI。在PCI后4小时至5天,使用隐蔽,分层和封闭的基于网络的中央随机分组将参与者随机分配(1:1)到edoxaban(每天一次60 mg)加上12个月的P2Y12抑制剂或维生素K拮抗剂(VKA)与P2Y12抑制剂和阿司匹林(每天一次100毫克,1-12个月)。如果存在一种或多种因素(肌酐清除率15-50 mL / min,体重≤60kg或同时使用指定的强力P-糖蛋白抑制剂),则edoxaban的剂量可减少至每天30 mg。主要终点是12个月内发生的主要或临床相关的非主要(CRNM)出血的复合症状。在意向性治疗人群中进行了初步分析,并评估了接受至少一种剂量指定研究药物的所有患者的安全性。该临床试验已在ClinicalTrials.gov上注册,编号为NCT02866175,不对新参与者开放,并已完成随访。结果从2017年2月24日到2018年5月7日,共纳入1506例患者并随机分配至edoxaban方案(n = 751)或VKA方案(n = 755)。从PCI到随机分组的中位时间为45·1 h(IQR 22·2-76·2)。751例依多沙班方案患者(年事件发生率20·7%)中有128例(17%)发生重大或CRNM出血事件,而VKA 755例患者(事件事件发生率25·6%)中的152例(20%)发生养生 危险比0·83(95%CI 0·65-1·05;非劣等p = 0·0010,边际危险比1·20;优势p = 0·1154)。解释在有PCI的房颤患者中,基于依多沙班的治疗方案与基于VKA的治疗方案相比,出血效果不差,缺血事件无明显差异。资助第一三共。边际风险比1·20;p = 0·1154为优势)。解释在有PCI的房颤患者中,基于依多沙班的治疗方案与基于VKA的治疗方案相比,出血效果不差,缺血事件无明显差异。资助第一三共。边际风险比1·20;p = 0·1154为优势)。解释在有PCI的房颤患者中,基于依多沙班的治疗方案与基于VKA的治疗方案相比,出血效果不差,缺血事件无明显差异。资助第一三共。
更新日期:2019-10-12
中文翻译:

在房颤患者成功完成冠状动脉支架置入术后,使用基于依多沙班和基于维生素K拮抗剂的抗血栓治疗方案(ENTRUST-AF PCI):一项随机,开放标签的3b期试验。
背景我们旨在评估依多沙班联合P2Y12抑制在经皮冠状动脉介入治疗(PCI)的房颤患者中的安全性。方法ENTRUST-AF PCI是一项随机,多中心,开放性,非劣效性3b期临床试验,其掩盖的结果评估在18个国家的186个地点进行。患有房颤的患者需要口服抗凝药物,年龄至少18岁,并且成功获得了可用于稳定冠状动脉疾病或急性冠状动脉综合征的PCI。在PCI后4小时至5天,使用隐蔽,分层和封闭的基于网络的中央随机分组将参与者随机分配(1:1)到edoxaban(每天一次60 mg)加上12个月的P2Y12抑制剂或维生素K拮抗剂(VKA)与P2Y12抑制剂和阿司匹林(每天一次100毫克,1-12个月)。如果存在一种或多种因素(肌酐清除率15-50 mL / min,体重≤60kg或同时使用指定的强力P-糖蛋白抑制剂),则edoxaban的剂量可减少至每天30 mg。主要终点是12个月内发生的主要或临床相关的非主要(CRNM)出血的复合症状。在意向性治疗人群中进行了初步分析,并评估了接受至少一种剂量指定研究药物的所有患者的安全性。该临床试验已在ClinicalTrials.gov上注册,编号为NCT02866175,不对新参与者开放,并已完成随访。结果从2017年2月24日到2018年5月7日,共纳入1506例患者并随机分配至edoxaban方案(n = 751)或VKA方案(n = 755)。从PCI到随机分组的中位时间为45·1 h(IQR 22·2-76·2)。751例依多沙班方案患者(年事件发生率20·7%)中有128例(17%)发生重大或CRNM出血事件,而VKA 755例患者(事件事件发生率25·6%)中的152例(20%)发生养生 危险比0·83(95%CI 0·65-1·05;非劣等p = 0·0010,边际危险比1·20;优势p = 0·1154)。解释在有PCI的房颤患者中,基于依多沙班的治疗方案与基于VKA的治疗方案相比,出血效果不差,缺血事件无明显差异。资助第一三共。边际风险比1·20;p = 0·1154为优势)。解释在有PCI的房颤患者中,基于依多沙班的治疗方案与基于VKA的治疗方案相比,出血效果不差,缺血事件无明显差异。资助第一三共。边际风险比1·20;p = 0·1154为优势)。解释在有PCI的房颤患者中,基于依多沙班的治疗方案与基于VKA的治疗方案相比,出血效果不差,缺血事件无明显差异。资助第一三共。











































 京公网安备 11010802027423号
京公网安备 11010802027423号