Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biodegradable polymer sirolimus-eluting stents versus durable polymer everolimus-eluting stents in patients with ST-segment elevation myocardial infarction (BIOSTEMI): a single-blind, prospective, randomised superiority trial
The Lancet ( IF 98.4 ) Pub Date : 2019-09-01 , DOI: 10.1016/s0140-6736(19)31877-x Juan F Iglesias , Olivier Muller , Dik Heg , Marco Roffi , David J Kurz , Igal Moarof , Daniel Weilenmann , Christoph Kaiser , Maxime Tapponnier , Stefan Stortecky , Sylvain Losdat , Eric Eeckhout , Marco Valgimigli , Ayodele Odutayo , Marcel Zwahlen , Peter Jüni , Stephan Windecker , Thomas Pilgrim
中文翻译:

ST段抬高型心肌梗死(BIOSTEMI)患者的生物可降解聚合物西罗莫司洗脱支架与耐用聚合物依维莫司洗脱支架的比较:一项单盲,前瞻性,随机性试验
更新日期:2019-10-04
The Lancet ( IF 98.4 ) Pub Date : 2019-09-01 , DOI: 10.1016/s0140-6736(19)31877-x Juan F Iglesias , Olivier Muller , Dik Heg , Marco Roffi , David J Kurz , Igal Moarof , Daniel Weilenmann , Christoph Kaiser , Maxime Tapponnier , Stefan Stortecky , Sylvain Losdat , Eric Eeckhout , Marco Valgimigli , Ayodele Odutayo , Marcel Zwahlen , Peter Jüni , Stephan Windecker , Thomas Pilgrim
|
Background
Newer-generation drug-eluting stents that combine ultrathin strut metallic platforms with biodegradable polymers might facilitate vascular healing and improve clinical outcomes in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention (PCI) compared with contemporary thin strut second-generation drug-eluting stents. We did a randomised clinical trial to investigate the safety and efficacy of ultrathin strut biodegradable polymer sirolimus-eluting stents versus thin strut durable polymer everolimus-eluting stents in patients with acute ST-segment elevation myocardial infarction (STEMI) undergoing primary PCI.Methods
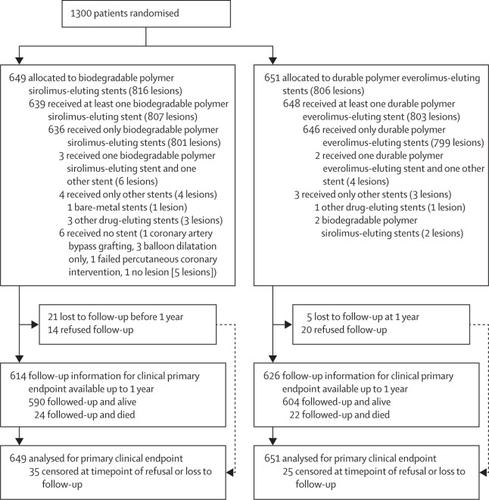
The BIOSTEMI trial was an investigator-initiated, multicentre, prospective, single-blind, randomised superiority trial at ten hospitals in Switzerland. Patients aged 18 years or older with acute STEMI who were referred for primary PCI were eligible to participate. Patients were randomly allocated (1:1) to either biodegradable polymer sirolimus-eluting stents or durable polymer everolimus-eluting stents. Central randomisation was done based on a computer-generated allocation sequence with variable block sizes of 2, 4, and 6, which was stratified by centre, diabetes status, and presence or absence of multivessel coronary artery disease, and concealed using a secure web-based system. Patients and treating physicians were aware of group allocations, whereas outcome assessors were masked to the allocated stent. The experimental stent (Orsiro; Biotronik; Bülach, Switzerland) consisted of an ultrathin strut cobalt–chromium metallic stent platform releasing sirolimus from a biodegradable polymer. The control stent (Xience Xpedition/Alpine; Abbott Vascular, Abbott Park, IL, USA) consisted of a thin strut cobalt–chromium stent platform that releases everolimus from a durable polymer. The primary endpoint was target lesion failure, a composite of cardiac death, target vessel myocardial reinfarction (Q-wave and non-Q-wave), and clinically-indicated target lesion revascularisation, within 12 months of the index procedure. All analyses were done with the individual participant as the unit of analysis and according to the intention-to-treat principle. The trial was registered with , number .Findings
Between April 26, 2016, and March 9, 2018, we randomly assigned 1300 patients (1623 lesions) with acute myocardial infarction to treatment with biodegradable polymer sirolimus-eluting stents (649 patients and 816 lesions) or durable polymer everolimus-eluting stents (651 patients and 806 lesions). At 12 months, follow-up data were available for 614 (95%) patients treated with biodegradable polymer sirolimus-eluting stents and 626 (96%) patients treated with durable polymer everolimus-eluting stents. The primary composite endpoint of target lesion failure occurred in 25 (4%) of 649 patients treated with biodegradable polymer sirolimus-eluting stents and 36 (6%) of 651 patients treated with durable polymer everolimus-eluting stents (difference −1·6 percentage points; rate ratio 0·59, 95% Bayesian credibility interval 0·37–0·94; posterior probability of superiority 0·986). Cardiac death, target vessel myocardial reinfarction, clinically-indicated target lesion revascularisation, and definite stent thrombosis were similar between the two treatment groups in the 12 months of follow-up.Interpretation
In patients with acute STEMI undergoing primary PCI, biodegradable polymer sirolimus-eluting stents were superior to durable polymer everolimus-eluting stents with respect to target lesion failure at 1 year. This difference was driven by reduced ischaemia-driven target lesion revascularisation in patients treated with biodegradable polymer sirolimus-eluting stents compared with durable polymer everolimus-eluting stents.Funding
Biotronik.中文翻译:

ST段抬高型心肌梗死(BIOSTEMI)患者的生物可降解聚合物西罗莫司洗脱支架与耐用聚合物依维莫司洗脱支架的比较:一项单盲,前瞻性,随机性试验











































 京公网安备 11010802027423号
京公网安备 11010802027423号