Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ticagrelor in patients with diabetes and stable coronary artery disease with a history of previous percutaneous coronary intervention (THEMIS-PCI): a phase 3, placebo-controlled, randomised trial.
The Lancet ( IF 168.9 ) Pub Date : 2019-09-01 , DOI: 10.1016/s0140-6736(19)31887-2 Deepak L Bhatt 1 , Philippe Gabriel Steg 2 , Shamir R Mehta 3 , Lawrence A Leiter 4 , Tabassome Simon 5 , Kim Fox 6 , Claes Held 7 , Marielle Andersson 8 , Anders Himmelmann 8 , Wilhelm Ridderstråle 8 , Jersey Chen 9 , Yang Song 10 , Rafael Diaz 11 , Shinya Goto 12 , Stefan K James 13 , Kausik K Ray 14 , Alexander N Parkhomenko 15 , Mikhail N Kosiborod 16 , Darren K McGuire 17 , Robert A Harrington 18 ,
中文翻译:

具有既往经皮冠状动脉介入治疗史(THEMIS-PCI)的糖尿病和稳定冠状动脉疾病患者的替卡格雷治疗:一项3期,安慰剂对照,随机试验。
更新日期:2019-09-26
The Lancet ( IF 168.9 ) Pub Date : 2019-09-01 , DOI: 10.1016/s0140-6736(19)31887-2 Deepak L Bhatt 1 , Philippe Gabriel Steg 2 , Shamir R Mehta 3 , Lawrence A Leiter 4 , Tabassome Simon 5 , Kim Fox 6 , Claes Held 7 , Marielle Andersson 8 , Anders Himmelmann 8 , Wilhelm Ridderstråle 8 , Jersey Chen 9 , Yang Song 10 , Rafael Diaz 11 , Shinya Goto 12 , Stefan K James 13 , Kausik K Ray 14 , Alexander N Parkhomenko 15 , Mikhail N Kosiborod 16 , Darren K McGuire 17 , Robert A Harrington 18 ,
Affiliation
|
Background
Patients with stable coronary artery disease and diabetes with previous percutaneous coronary intervention (PCI), particularly those with previous stenting, are at high risk of ischaemic events. These patients are generally treated with aspirin. In this trial, we aimed to investigate if these patients would benefit from treatment with aspirin plus ticagrelor.Methods
The Effect of Ticagrelor on Health Outcomes in diabEtes Mellitus patients Intervention Study (THEMIS) was a phase 3 randomised, double-blinded, placebo-controlled trial, done in 1315 sites in 42 countries. Patients were eligible if 50 years or older, with type 2 diabetes, receiving anti-hyperglycaemic drugs for at least 6 months, with stable coronary artery disease, and one of three other mutually non-exclusive criteria: a history of previous PCI or of coronary artery bypass grafting, or documentation of angiographic stenosis of 50% or more in at least one coronary artery. Eligible patients were randomly assigned (1:1) to either ticagrelor or placebo, by use of an interactive voice-response or web-response system. The THEMIS-PCI trial comprised a prespecified subgroup of patients with previous PCI. The primary efficacy outcome was a composite of cardiovascular death, myocardial infarction, or stroke (measured in the intention-to-treat population).Findings
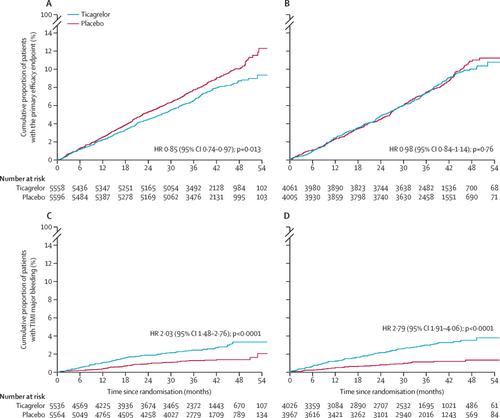
Between Feb 17, 2014, and May 24, 2016, 11 154 patients (58% of the overall THEMIS trial) with a history of previous PCI were enrolled in the THEMIS-PCI trial. Median follow-up was 3·3 years (IQR 2·8–3·8). In the previous PCI group, fewer patients receiving ticagrelor had a primary efficacy outcome event than in the placebo group (404 [7·3%] of 5558 vs 480 [8·6%] of 5596; HR 0·85 [95% CI 0·74–0·97], p=0·013). The same effect was not observed in patients without PCI (p=0·76, p interaction=0·16). The proportion of patients with cardiovascular death was similar in both treatment groups (174 [3·1%] with ticagrelor vs 183 (3·3%) with placebo; HR 0·96 [95% CI 0·78–1·18], p=0·68), as well as all-cause death (282 [5·1%] vs 323 [5·8%]; 0·88 [0·75–1·03], p=0·11). TIMI major bleeding occurred in 111 (2·0%) of 5536 patients receiving ticagrelor and 62 (1·1%) of 5564 patients receiving placebo (HR 2·03 [95% CI 1·48–2·76], p<0·0001), and fatal bleeding in 6 (0·1%) of 5536 patients with ticagrelor and 6 (0·1%) of 5564 with placebo (1·13 [0·36–3·50], p=0·83). Intracranial haemorrhage occurred in 33 (0·6%) and 31 (0·6%) patients (1·21 [0·74–1·97], p=0·45). Ticagrelor improved net clinical benefit: 519/5558 (9·3%) versus 617/5596 (11·0%), HR=0·85, 95% CI 0·75–0·95, p=0·005, in contrast to patients without PCI where it did not, p interaction=0·012. Benefit was present irrespective of time from most recent PCI.Interpretation
In patients with diabetes, stable coronary artery disease, and previous PCI, ticagrelor added to aspirin reduced cardiovascular death, myocardial infarction, and stroke, although with increased major bleeding. In that large, easily identified population, ticagrelor provided a favourable net clinical benefit (more than in patients without history of PCI). This effect shows that long-term therapy with ticagrelor in addition to aspirin should be considered in patients with diabetes and a history of PCI who have tolerated antiplatelet therapy, have high ischaemic risk, and low bleeding risk.Funding
AstraZeneca.中文翻译:

具有既往经皮冠状动脉介入治疗史(THEMIS-PCI)的糖尿病和稳定冠状动脉疾病患者的替卡格雷治疗:一项3期,安慰剂对照,随机试验。



























 京公网安备 11010802027423号
京公网安备 11010802027423号