npj Regenerative Medicine ( IF 6.4 ) Pub Date : 2019-08-27 , DOI: 10.1038/s41536-019-0081-8 Tina Guanting Qiu
|
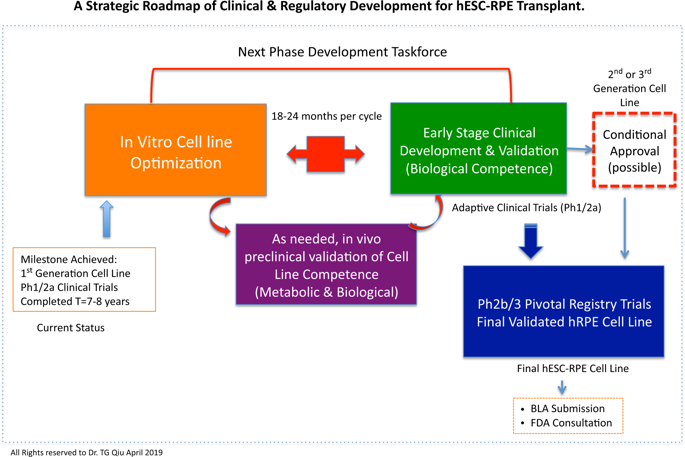
The use of human embryonic stem cell (hESC)-derived Retinal Pigment Epithelium (RPE) transplants has advanced dramatically in different forms for clinical application in macular degeneration. This review focuses on the first generation of hESC-RPE cell line, named as “MA09-hRPE” by Astellas Institute of Regenerative Medicine (AIRM), and its therapeutic application in human, which evaluated the safety and efficacy of MA09-hRPE cell line transplanted in patients with macular degeneration. This project marks the first milestone in overcoming ethical hurdles and oncogenic safety concerns associated with the use of an embryonic stem cell-derived line. Through in-depth, evidence-based analysis of the MA09-hRPE cell line, along with other hESC-RPE cell lines, this review aims to draw attention to the key technical challenges pertinent to the generation of a biologically competent hESC-RPE cell line and distill the four key prognostic factors residing in the host retina, which concurrently determine the outcomes of clinical efficacy and visual benefits. Given that the technology is still at its infancy for human use, a new clinical regulatory path could aid in cell line validation through small cohort, adaptive clinical trials to accelerate product development toward commercialization. These strategic insights will be invaluable to help both academia and industry, collaboratively shorten the steep learning curve, and reduce large development expenditures spent on unnecessary lengthy clinical trials.
中文翻译:

黄斑变性中人胚胎干细胞源性视网膜色素上皮细胞(MA09-hRPE)的移植
人类胚胎干细胞(hESC)衍生的视网膜色素上皮(RPE)移植的使用已经以不同形式显着发展,用于黄斑变性的临床应用。这篇综述着重于第一代hESC-RPE细胞系,被Astellas再生医学研究所(AIRM)命名为“ MA09-hRPE”,及其在人体中的治疗应用,从而评估了MA09-hRPE细胞系的安全性和有效性。黄斑变性患者移植。该项目标志着克服与胚胎干细胞衍生品系的使用相关的道德障碍和致癌安全性关注的第一个里程碑。通过对MA09-hRPE细胞系以及其他hESC-RPE细胞系进行深入的,基于证据的分析,这篇综述旨在引起人们对与具有生物学能力的hESC-RPE细胞系产生相关的关键技术挑战的关注,并提炼驻留在宿主视网膜中的四个关键预后因素,这些因素同时决定了临床疗效和视觉效果。鉴于该技术仍处于供人类使用的初期阶段,一条新的临床监管途径可以通过小型队列,适应性临床试验来帮助细胞系验证,从而加速产品开发的商业化进程。这些战略见解对于帮助学术界和工业界,协同缩短陡峭的学习曲线并减少不必要的冗长的临床试验所花费的大量开发支出将具有不可估量的价值。









































 京公网安备 11010802027423号
京公网安备 11010802027423号