当前位置:
X-MOL 学术
›
Fungal Genet. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role and dynamics of an agmatinase-like protein (AGM-1) in Neurospora crassa.
Fungal Genetics and Biology ( IF 2.4 ) Pub Date : 2019-08-26 , DOI: 10.1016/j.fgb.2019.103264 Luis L Pérez-Mozqueda 1 , Rafael Vazquez-Duhalt 2 , Ernestina Castro-Longoria 1
Fungal Genetics and Biology ( IF 2.4 ) Pub Date : 2019-08-26 , DOI: 10.1016/j.fgb.2019.103264 Luis L Pérez-Mozqueda 1 , Rafael Vazquez-Duhalt 2 , Ernestina Castro-Longoria 1
Affiliation
|
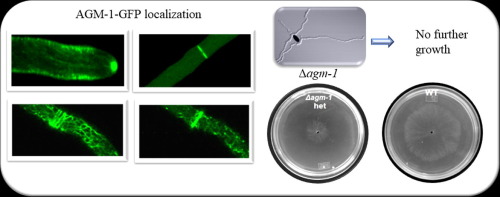
Agmatinase is known as a metalloenzyme which hydrolyzes agmatine to produce urea and putrescine, being crucial in the alternative pathway to produce polyamines. In this study, an agmatinase-like protein (AGM-1) (NCU 01348) in the filamentous fungus Neurospora crassa is reported. Purified AGM-1 from N. crassa displays enzymatic activity hydrolyzing agmatine; therefore, it can be considered as an agmatinase-like protein. However, its role in the alternative pathway to produce polyamines apparently is not its main function since only a slight reduction of polyamines concentration was detected in the Δagm-1 het strain. Moreover, the null mutant Δagm-1 (homokaryon strain) was unable to grow and the deficiency of agm-1 in the heterokaryon strain provoked a decrease in elongation rate, conidia and biomass production, despite of having de constitutive pathway via the ornithine decarboxylase (ODC). Additionally, mature hyphae of the Δagm-1 het strain presented unusual apical branching and a disorganized Spitzenkörper (Spk). Trying to reveal the role of AGM-1in N. crassa, the protein was tagged with GFP and interestingly the dynamics and intracellular localization of AGM-1 closely resembles the F-actin population. This finding was further examined in order to elucidate if AGM-1is in a close association with F-actin. Since polyamines, among them agmatine, have been reported to act as stabilizers of actin filaments, we evaluated in vitro G-actin polymerization in the presence of agmatine and the effect of purified AGM-1 from N. crassa on these polymerized actin filaments. It was found that polymerization of actin filaments increases in the presence of agmatine and the addition of purified AGM-1 from N. crassa depolymerizes these actin filaments. Also, it was determined that an intact substrate binding site of the enzyme is necessary for the localization pattern of the native AGM-1. These results suggest that in N. crassa AGM-1 has a close association with the F-actin population via its substrate agmatine, playing an essential role during cell development.
中文翻译:

芥末酶样蛋白(AGM-1)在神经孢菌中的作用和动力学。
胍丁胺酶被称为金属酶,其水解胍丁胺以产生尿素和腐胺,这在产生多胺的替代途径中是至关重要的。在这项研究中,报道了丝状真菌Neurospora crassa中的一种类固胺酶样蛋白(AGM-1)(NCU 01348)。来自N. crassa的纯化的AGM-1具有水解胍丁胺的酶活性。因此,它可以被认为是一种胍基酶样蛋白。但是,它在生产多胺的替代途径中的作用显然不是其主要功能,因为在Δagm-1het菌株中仅检测到多胺浓度略有降低。此外,无效突变体Δagm-1(纯核细胞株)无法生长,异核体株中agm-1的缺乏导致伸长率,分生孢子和生物量产生下降,尽管具有通过鸟氨酸脱羧酶(ODC)的构成性途径。此外,Δagm-1het菌株的成熟菌丝表现出不寻常的根尖分支和无序的Spitzenkörper(Spk)。为了揭示AGM-1在猪笼草中的作用,用GFP标记了该蛋白,有趣的是,AGM-1的动力学和细胞内定位与F-肌动蛋白种群非常相似。为了阐明AGM-1是否与F-肌动蛋白密切相关,进一步检查了该发现。由于据报道聚胺(其中包括胍丁胺)可作为肌动蛋白丝的稳定剂,因此我们在存在胍丁胺的情况下评估了体外G-肌动蛋白聚合反应,以及从景天猪笼草中提纯的AGM-1对这些聚合的肌动蛋白丝的影响。已经发现,在存在胍丁胺的情况下,肌动蛋白丝的聚合增加,并且从猪笼草中纯化的AGM-1的添加使这些肌动蛋白丝解聚。此外,已确定完整的酶底物结合位点对于天然AGM-1的定位模式是必需的。这些结果表明,在猪笼草中,AGM-1通过其底物胍丁胺与F-肌动蛋白群体密切相关,在细胞发育过程中起着至关重要的作用。
更新日期:2019-08-26
中文翻译:

芥末酶样蛋白(AGM-1)在神经孢菌中的作用和动力学。
胍丁胺酶被称为金属酶,其水解胍丁胺以产生尿素和腐胺,这在产生多胺的替代途径中是至关重要的。在这项研究中,报道了丝状真菌Neurospora crassa中的一种类固胺酶样蛋白(AGM-1)(NCU 01348)。来自N. crassa的纯化的AGM-1具有水解胍丁胺的酶活性。因此,它可以被认为是一种胍基酶样蛋白。但是,它在生产多胺的替代途径中的作用显然不是其主要功能,因为在Δagm-1het菌株中仅检测到多胺浓度略有降低。此外,无效突变体Δagm-1(纯核细胞株)无法生长,异核体株中agm-1的缺乏导致伸长率,分生孢子和生物量产生下降,尽管具有通过鸟氨酸脱羧酶(ODC)的构成性途径。此外,Δagm-1het菌株的成熟菌丝表现出不寻常的根尖分支和无序的Spitzenkörper(Spk)。为了揭示AGM-1在猪笼草中的作用,用GFP标记了该蛋白,有趣的是,AGM-1的动力学和细胞内定位与F-肌动蛋白种群非常相似。为了阐明AGM-1是否与F-肌动蛋白密切相关,进一步检查了该发现。由于据报道聚胺(其中包括胍丁胺)可作为肌动蛋白丝的稳定剂,因此我们在存在胍丁胺的情况下评估了体外G-肌动蛋白聚合反应,以及从景天猪笼草中提纯的AGM-1对这些聚合的肌动蛋白丝的影响。已经发现,在存在胍丁胺的情况下,肌动蛋白丝的聚合增加,并且从猪笼草中纯化的AGM-1的添加使这些肌动蛋白丝解聚。此外,已确定完整的酶底物结合位点对于天然AGM-1的定位模式是必需的。这些结果表明,在猪笼草中,AGM-1通过其底物胍丁胺与F-肌动蛋白群体密切相关,在细胞发育过程中起着至关重要的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号