当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-step pathway to selenoisobenzofuran-1(3H)-imine derivatives through highly selective selenocyclization of olefinic amides with benzeneselenyl chloride†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1039/c8ob00179k Xiwang Li 1, 2, 3, 4, 5 , Pei He 1, 2, 3, 4, 5 , Hai-Bing Zhou 1, 2, 3, 4, 5 , Chune Dong 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1039/c8ob00179k Xiwang Li 1, 2, 3, 4, 5 , Pei He 1, 2, 3, 4, 5 , Hai-Bing Zhou 1, 2, 3, 4, 5 , Chune Dong 1, 2, 3, 4, 5
Affiliation
|
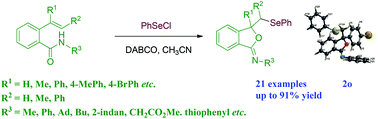
A 1,4-diazabicyclo[2.2.2]octane (DABCO) catalyzed selenocyclization of olefinic amides was achieved under mild reaction conditions. The reaction formed various benzeneselenyl substituted isobenzofuran-1(3H)-imine derivatives in good yields. The product was determined using single-crystal X-ray analysis. For compound 2u, the relative stereochemistry was established on the basis of NOESY NMR studies.
中文翻译:

一步法途径selenoisobenzofuran-1(3 ħ)通过与氯化benzeneselenyl烯酰胺的高度选择性selenocyclization -亚胺衍生物†
在温和的反应条件下,实现了1,4-二氮杂双环[2.2.2]辛烷(DABCO)催化的烯烃酰胺的硒代环化反应。该反应以良好的产率形成了各种苯硒基取代的异苯并呋喃-1(3 H)-亚胺衍生物。使用单晶X射线分析确定产物。对于化合物2u,基于NOESY NMR研究确定了相对立体化学。
更新日期:2018-03-01
中文翻译:

一步法途径selenoisobenzofuran-1(3 ħ)通过与氯化benzeneselenyl烯酰胺的高度选择性selenocyclization -亚胺衍生物†
在温和的反应条件下,实现了1,4-二氮杂双环[2.2.2]辛烷(DABCO)催化的烯烃酰胺的硒代环化反应。该反应以良好的产率形成了各种苯硒基取代的异苯并呋喃-1(3 H)-亚胺衍生物。使用单晶X射线分析确定产物。对于化合物2u,基于NOESY NMR研究确定了相对立体化学。









































 京公网安备 11010802027423号
京公网安备 11010802027423号