Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): a pragmatic, cluster-randomised trial.
The Lancet ( IF 98.4 ) Pub Date : 2019-08-24 , DOI: 10.1016/s0140-6736(19)31791-x Gholamreza Roshandel 1 , Masoud Khoshnia 1 , Hossein Poustchi 2 , Karla Hemming 3 , Farin Kamangar 4 , Abdolsamad Gharavi 5 , Mohammad Reza Ostovaneh 5 , Alireza Nateghi 5 , Masoud Majed 5 , Behrooz Navabakhsh 5 , Shahin Merat 6 , Akram Pourshams 2 , Mahdi Nalini 5 , Fatemeh Malekzadeh 5 , Masoumeh Sadeghi 7 , Noushin Mohammadifard 8 , Nizal Sarrafzadegan 9 , Mohammad Naemi-Tabiei 10 , Abdolreza Fazel 10 , Paul Brennan 11 , Arash Etemadi 12 , Paolo Boffetta 13 , Neil Thomas 3 , Tom Marshall 3 , Kar Keung Cheng 3 , Reza Malekzadeh 14
The Lancet ( IF 98.4 ) Pub Date : 2019-08-24 , DOI: 10.1016/s0140-6736(19)31791-x Gholamreza Roshandel 1 , Masoud Khoshnia 1 , Hossein Poustchi 2 , Karla Hemming 3 , Farin Kamangar 4 , Abdolsamad Gharavi 5 , Mohammad Reza Ostovaneh 5 , Alireza Nateghi 5 , Masoud Majed 5 , Behrooz Navabakhsh 5 , Shahin Merat 6 , Akram Pourshams 2 , Mahdi Nalini 5 , Fatemeh Malekzadeh 5 , Masoumeh Sadeghi 7 , Noushin Mohammadifard 8 , Nizal Sarrafzadegan 9 , Mohammad Naemi-Tabiei 10 , Abdolreza Fazel 10 , Paul Brennan 11 , Arash Etemadi 12 , Paolo Boffetta 13 , Neil Thomas 3 , Tom Marshall 3 , Kar Keung Cheng 3 , Reza Malekzadeh 14
Affiliation
|
BACKGROUND
A fixed-dose combination therapy (polypill strategy) has been proposed as an approach to reduce the burden of cardiovascular disease, especially in low-income and middle-income countries (LMICs). The PolyIran study aimed to assess the effectiveness and safety of a four-component polypill including aspirin, atorvastatin, hydrochlorothiazide, and either enalapril or valsartan for primary and secondary prevention of cardiovascular disease.
METHODS
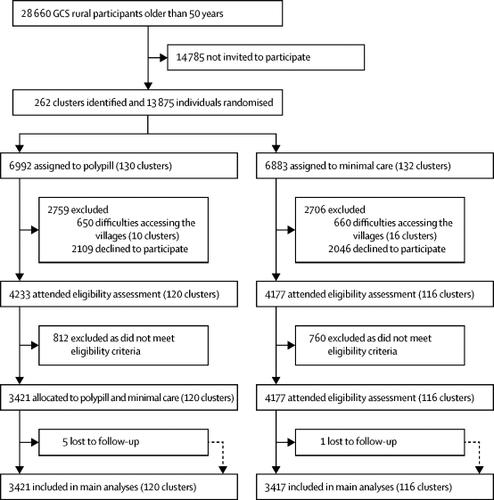
The PolyIran study was a two-group, pragmatic, cluster-randomised trial nested within the Golestan Cohort Study (GCS), a cohort study with 50 045 participants aged 40-75 years from the Golestan province in Iran. Clusters (villages) were randomly allocated (1:1) to either a package of non-pharmacological preventive interventions alone (minimal care group) or together with a once-daily polypill tablet (polypill group). Randomisation was stratified by three districts (Gonbad, Aq-Qala, and Kalaleh), with the village as the unit of randomisation. We used a balanced randomisation algorithm, considering block sizes of 20 and balancing for cluster size or natural log of the cluster size (depending on the skewness within strata). Randomisation was done at a fixed point in time (Jan 18, 2011) by statisticians at the University of Birmingham (Birmingham, UK), independent of the local study team. The non-pharmacological preventive interventions (including educational training about healthy lifestyle-eg, healthy diet with low salt, sugar, and fat content, exercise, weight control, and abstinence from smoking and opium) were delivered by the PolyIran field visit team at months 3 and 6, and then every 6 months thereafter. Two formulations of polypill tablet were used in this study. Participants were first prescribed polypill one (hydrochlorothiazide 12·5 mg, aspirin 81 mg, atorvastatin 20 mg, and enalapril 5 mg). Participants who developed cough during follow-up were switched by a trained study physician to polypill two, which included valsartan 40 mg instead of enalapril 5 mg. Participants were followed up for 60 months. The primary outcome-occurrence of major cardiovascular events (including hospitalisation for acute coronary syndrome, fatal myocardial infarction, sudden death, heart failure, coronary artery revascularisation procedures, and non-fatal and fatal stroke)-was centrally assessed by the GCS follow-up team, who were masked to allocation status. We did intention-to-treat analyses by including all participants who met eligibility criteria in the two study groups. The trial was registered with ClinicalTrials.gov, number NCT01271985.
FINDINGS
Between Feb 22, 2011, and April 15, 2013, we enrolled 6838 individuals into the study-3417 (in 116 clusters) in the minimal care group and 3421 (in 120 clusters) in the polypill group. 1761 (51·5%) of 3421 participants in the polypill group were women, as were 1679 (49·1%) of 3417 participants in the minimal care group. Median adherence to polypill tablets was 80·5% (IQR 48·5-92·2). During follow-up, 301 (8·8%) of 3417 participants in the minimal care group had major cardiovascular events compared with 202 (5·9%) of 3421 participants in the polypill group (adjusted hazard ratio [HR] 0·66, 95% CI 0·55-0·80). We found no statistically significant interaction with the presence (HR 0·61, 95% CI 0·49-0·75) or absence of pre-existing cardiovascular disease (0·80; 0·51-1·12; pinteraction=0·19). When restricted to participants in the polypill group with high adherence, the reduction in the risk of major cardiovascular events was even greater compared with the minimal care group (adjusted HR 0·43, 95% CI 0·33-0·55). The frequency of adverse events was similar between the two study groups. 21 intracranial haemorrhages were reported during the 5 years of follow-up-ten participants in the polypill group and 11 participants in the minimal care group. There were 13 physician-confirmed diagnoses of upper gastrointestinal bleeding in the polypill group and nine in the minimal care group.
INTERPRETATION
Use of polypill was effective in preventing major cardiovascular events. Medication adherence was high and adverse event numbers were low. The polypill strategy could be considered as an additional effective component in controlling cardiovascular diseases, especially in LMICs.
FUNDING
Tehran University of Medical Sciences, Barakat Foundation, and Alborz Darou.
中文翻译:

复方丸对心血管疾病一级和二级预防的有效性(PolyIran):一项务实的整群随机试验。
背景固定剂量联合疗法(多药丸策略)已被提议作为减轻心血管疾病负担的方法,特别是在低收入和中等收入国家(LMIC)。 PolyIran 研究旨在评估四成分复方药(包括阿司匹林、阿托伐他汀、氢氯噻嗪以及依那普利或缬沙坦)用于心血管疾病一级和二级预防的有效性和安全性。方法 PolyIran 研究是一项嵌套在 Golestan 队列研究 (GCS) 内的一项两组、务实、整群随机试验,该队列研究有来自伊朗 Golestan 省的 50 045 名年龄在 40-75 岁之间的参与者。集群(村庄)被随机分配(1:1),要么单独接受非药物预防干预措施(最低限度护理组),要么与每日一次的复方药片一起服用(复方药组)。随机化按三个区(贡巴德、阿克卡拉和卡拉莱)进行分层,以村庄为随机化单位。我们使用平衡随机化算法,考虑 20 的块大小并平衡簇大小或簇大小的自然对数(取决于层内的偏度)。随机化是由伯明翰大学(英国伯明翰)的统计学家在固定时间点(2011 年 1 月 18 日)完成的,独立于当地研究团队。聚伊朗实地考察小组在几个月内提供了非药物预防干预措施(包括有关健康生活方式的教育培训,例如低盐、低糖和脂肪含量的健康饮食、锻炼、控制体重以及戒烟和鸦片) 3 和 6 次,此后每 6 个月一次。本研究使用了两种配方的聚丸片剂。 首先给参与者开了复方药一(氢氯噻嗪 12·5 mg、阿司匹林 81 mg、阿托伐他汀 20 mg 和依那普利 5 mg)。在随访期间出现咳嗽的参与者被训练有素的研究医生转为复方二号复方药,其中包括 40 毫克缬沙坦,而不是 5 毫克依那普利。参与者被随访了 60 个月。主要结局——主要心血管事件(包括急性冠脉综合征住院、致命性心肌梗死、猝死、心力衰竭、冠状动脉血运重建手术以及非致命性和致命性中风)的发生——由 GCS 随访进行集中评估团队,他们不知道分配状态。我们通过纳入两个研究组中符合资格标准的所有参与者来进行意向治疗分析。该试验已在 ClinicalTrials.gov 注册,注册号为 NCT01271985。结果 2011 年 2 月 22 日至 2013 年 4 月 15 日期间,我们招募了 6838 名受试者参加研究,其中 3417 名受试者(分为 116 个集群)属于最低限度护理组,3421 名受试者(分为 120 个集群)属于复方药组。复方药组的 3421 名参与者中有 1761 名 (51·5%) 是女性,最低限度护理组的 3417 名参与者中有 1679 名 (49·1%) 是女性。聚丸片的中位依从率为 80·5% (IQR 48·5-92·2)。随访期间,最低限度护理组 3417 名参与者中有 301 名 (8·8%) 发生重大心血管事件,而复方药组 3421 名参与者中有 202 名 (5·9%) 发生重大心血管事件(调整后风险比 [HR] 0·66 ,95% CI 0·55-0·80)。我们发现与是否存在心血管疾病(HR 0·61,95% CI 0·49-0·75)或不存在(0·80;0·51-1·12;pinteraction = 0)之间没有统计学上显着的相互作用。 ·19)。 当仅限于高依从性的复方药组参与者时,与最低限度护理组相比,主要心血管事件风险的降低甚至更大(调整后的 HR 0·43,95% CI 0·33-0·55)。两个研究组之间的不良事件发生频率相似。在对复方药组的 10 名参与者和最低限度护理组的 11 名参与者进行 5 年随访期间,报告了 21 例颅内出血。复方避孕药组有 13 例经医生确诊为上消化道出血,最低限度护理组有 9 例。解释 使用复方丸可有效预防主要心血管事件。药物依从性高,不良事件发生率低。复方药策略可被视为控制心血管疾病的额外有效组成部分,尤其是在中低收入国家。资助德黑兰医科大学、巴拉卡特基金会和厄尔布尔士达鲁。
更新日期:2019-08-23
中文翻译:

复方丸对心血管疾病一级和二级预防的有效性(PolyIran):一项务实的整群随机试验。
背景固定剂量联合疗法(多药丸策略)已被提议作为减轻心血管疾病负担的方法,特别是在低收入和中等收入国家(LMIC)。 PolyIran 研究旨在评估四成分复方药(包括阿司匹林、阿托伐他汀、氢氯噻嗪以及依那普利或缬沙坦)用于心血管疾病一级和二级预防的有效性和安全性。方法 PolyIran 研究是一项嵌套在 Golestan 队列研究 (GCS) 内的一项两组、务实、整群随机试验,该队列研究有来自伊朗 Golestan 省的 50 045 名年龄在 40-75 岁之间的参与者。集群(村庄)被随机分配(1:1),要么单独接受非药物预防干预措施(最低限度护理组),要么与每日一次的复方药片一起服用(复方药组)。随机化按三个区(贡巴德、阿克卡拉和卡拉莱)进行分层,以村庄为随机化单位。我们使用平衡随机化算法,考虑 20 的块大小并平衡簇大小或簇大小的自然对数(取决于层内的偏度)。随机化是由伯明翰大学(英国伯明翰)的统计学家在固定时间点(2011 年 1 月 18 日)完成的,独立于当地研究团队。聚伊朗实地考察小组在几个月内提供了非药物预防干预措施(包括有关健康生活方式的教育培训,例如低盐、低糖和脂肪含量的健康饮食、锻炼、控制体重以及戒烟和鸦片) 3 和 6 次,此后每 6 个月一次。本研究使用了两种配方的聚丸片剂。 首先给参与者开了复方药一(氢氯噻嗪 12·5 mg、阿司匹林 81 mg、阿托伐他汀 20 mg 和依那普利 5 mg)。在随访期间出现咳嗽的参与者被训练有素的研究医生转为复方二号复方药,其中包括 40 毫克缬沙坦,而不是 5 毫克依那普利。参与者被随访了 60 个月。主要结局——主要心血管事件(包括急性冠脉综合征住院、致命性心肌梗死、猝死、心力衰竭、冠状动脉血运重建手术以及非致命性和致命性中风)的发生——由 GCS 随访进行集中评估团队,他们不知道分配状态。我们通过纳入两个研究组中符合资格标准的所有参与者来进行意向治疗分析。该试验已在 ClinicalTrials.gov 注册,注册号为 NCT01271985。结果 2011 年 2 月 22 日至 2013 年 4 月 15 日期间,我们招募了 6838 名受试者参加研究,其中 3417 名受试者(分为 116 个集群)属于最低限度护理组,3421 名受试者(分为 120 个集群)属于复方药组。复方药组的 3421 名参与者中有 1761 名 (51·5%) 是女性,最低限度护理组的 3417 名参与者中有 1679 名 (49·1%) 是女性。聚丸片的中位依从率为 80·5% (IQR 48·5-92·2)。随访期间,最低限度护理组 3417 名参与者中有 301 名 (8·8%) 发生重大心血管事件,而复方药组 3421 名参与者中有 202 名 (5·9%) 发生重大心血管事件(调整后风险比 [HR] 0·66 ,95% CI 0·55-0·80)。我们发现与是否存在心血管疾病(HR 0·61,95% CI 0·49-0·75)或不存在(0·80;0·51-1·12;pinteraction = 0)之间没有统计学上显着的相互作用。 ·19)。 当仅限于高依从性的复方药组参与者时,与最低限度护理组相比,主要心血管事件风险的降低甚至更大(调整后的 HR 0·43,95% CI 0·33-0·55)。两个研究组之间的不良事件发生频率相似。在对复方药组的 10 名参与者和最低限度护理组的 11 名参与者进行 5 年随访期间,报告了 21 例颅内出血。复方避孕药组有 13 例经医生确诊为上消化道出血,最低限度护理组有 9 例。解释 使用复方丸可有效预防主要心血管事件。药物依从性高,不良事件发生率低。复方药策略可被视为控制心血管疾病的额外有效组成部分,尤其是在中低收入国家。资助德黑兰医科大学、巴拉卡特基金会和厄尔布尔士达鲁。











































 京公网安备 11010802027423号
京公网安备 11010802027423号