Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthetic approach for optically active polymers through the combination of asymmetric chirogenic polymerization and postpolymerization modification
Polymer Journal ( IF 2.3 ) Pub Date : 2019-08-22 , DOI: 10.1038/s41428-019-0248-6 Naoya Kanbayashi
Polymer Journal ( IF 2.3 ) Pub Date : 2019-08-22 , DOI: 10.1038/s41428-019-0248-6 Naoya Kanbayashi
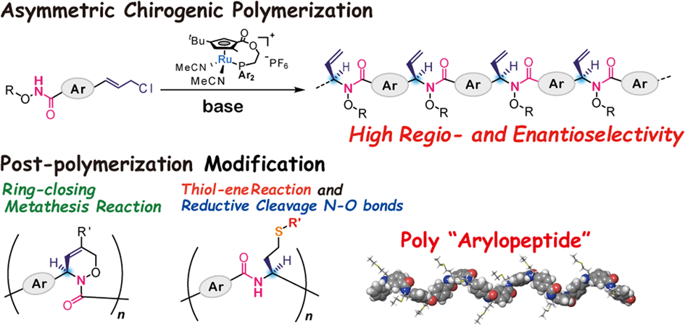
|
A new type of asymmetric chirogenic polymerization by asymmetric allylic substitution catalyzed by planar–chiral ruthenium complexes was designed. The polymerization systems function in a highly stereoselective manner to afford optically active polymers with high selectivity. The asymmetric carbon in the main chain is precisely controlled. Each monomer unit of the polymer has a potentially reactive terminal olefin, which can be used for further transformations. Optically active polymers bearing chiral cyclic architecture were prepared by a combination of asymmetric allylic substitution and ring-closing metathesis reaction employing the terminal olefin of the side chains. Additionally, the efficient introduction of a wide range of substituents into the side chain of the optically active polymer without any racemization has been made possible by using the thiol–ene reaction. Poly-N-alkoxyamides obtained by our asymmetric polymerization can be transformed into nonnatural polypeptides containing an aromatic ring on the peptide backbone, called a poly “arylopeptide”, through reductive cleavage of the N–O bond in N-alkoxyamide. The resulting polymer adopts a one-handed stable helical conformation in solution. A new type of asymmetric chirogenic polymerization by asymmetric allylic substitution catalyzed by planar–chiral ruthenium complexes was designed. The polymerization systems function in a highly stereoselective manner. The asymmetric carbon in the main chain is precisely controlled. Each monomer unit of the polymer has a potentially reactive terminal olefin, which can be used for further transformations, ring-closing metathesis and thiol–ene reaction. Additionally, the resulting polymer can be transformed poly “arylopeptide”, through reductive cleavage of the N–O bond in N-alkoxyamide, which adopts a one-handed stable helical conformation in solution.
中文翻译:

通过不对称手性聚合和聚合后改性相结合的光学活性聚合物的合成方法
设计了一种由平面-手性钌配合物催化的不对称烯丙基取代的新型不对称手性聚合。聚合体系以高度立体选择性的方式起作用,以提供具有高选择性的旋光聚合物。主链中的不对称碳原子得到精确控制。聚合物的每个单体单元都有一个潜在的反应性末端烯烃,可用于进一步的转化。具有手性环状结构的旋光聚合物是通过不对称烯丙基取代和使用侧链末端烯烃的闭环复分解反应的组合制备的。此外,通过使用硫醇 - 烯反应,可以在没有任何外消旋化的情况下有效地将范围广泛的取代基引入光学活性聚合物的侧链。通过不对称聚合获得的聚-N-烷氧基酰胺可以通过还原性裂解 N-烷氧基酰胺中的 N-O 键,转化为在肽主链上含有芳香环的非天然多肽,称为聚“芳基肽”。所得聚合物在溶液中采用单手稳定的螺旋构象。设计了一种由平面-手性钌配合物催化的不对称烯丙基取代的新型不对称手性聚合。聚合系统以高度立体选择性的方式起作用。主链中的不对称碳原子得到精确控制。聚合物的每个单体单元都有一个潜在的反应性末端烯烃,可用于进一步的转化、闭环复分解和硫醇-烯反应。此外,所得聚合物可以通过还原裂解 N-烷氧基酰胺中的 N-O 键转化为聚“芳肽”,在溶液中采用单手稳定的螺旋构象。
更新日期:2019-08-22
中文翻译:

通过不对称手性聚合和聚合后改性相结合的光学活性聚合物的合成方法
设计了一种由平面-手性钌配合物催化的不对称烯丙基取代的新型不对称手性聚合。聚合体系以高度立体选择性的方式起作用,以提供具有高选择性的旋光聚合物。主链中的不对称碳原子得到精确控制。聚合物的每个单体单元都有一个潜在的反应性末端烯烃,可用于进一步的转化。具有手性环状结构的旋光聚合物是通过不对称烯丙基取代和使用侧链末端烯烃的闭环复分解反应的组合制备的。此外,通过使用硫醇 - 烯反应,可以在没有任何外消旋化的情况下有效地将范围广泛的取代基引入光学活性聚合物的侧链。通过不对称聚合获得的聚-N-烷氧基酰胺可以通过还原性裂解 N-烷氧基酰胺中的 N-O 键,转化为在肽主链上含有芳香环的非天然多肽,称为聚“芳基肽”。所得聚合物在溶液中采用单手稳定的螺旋构象。设计了一种由平面-手性钌配合物催化的不对称烯丙基取代的新型不对称手性聚合。聚合系统以高度立体选择性的方式起作用。主链中的不对称碳原子得到精确控制。聚合物的每个单体单元都有一个潜在的反应性末端烯烃,可用于进一步的转化、闭环复分解和硫醇-烯反应。此外,所得聚合物可以通过还原裂解 N-烷氧基酰胺中的 N-O 键转化为聚“芳肽”,在溶液中采用单手稳定的螺旋构象。











































 京公网安备 11010802027423号
京公网安备 11010802027423号