当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel Pure αVβ3 Integrin Antagonists That Do Not Induce Receptor Extension, Prime the Receptor, or Enhance Angiogenesis at Low Concentrations.
ACS Pharmacology & Translational Science Pub Date : 2019-08-16 , DOI: 10.1021/acsptsci.9b00041 Jihong Li 1 , Yoshiyuki Fukase 2 , Yi Shang 3 , Wei Zou 4 , José M Muñoz-Félix 5 , Lorena Buitrago 1 , Johannes van Agthoven 6 , Yixiao Zhang 7 , Ryoma Hara 2 , Yuta Tanaka 2 , Rei Okamoto 2 , Takeshi Yasui 2 , Takashi Nakahata 2 , Toshihiro Imaeda 2 , Kazuyoshi Aso 2 , Yuchen Zhou 3 , Charles Locuson 8 , Dragana Nesic 1 , Mark Duggan 9 , Junichi Takagi 10 , Roger D Vaughan 11 , Thomas Walz 7 , Kairbaan Hodivala-Dilke 5 , Steven L Teitelbaum 4 , M Amin Arnaout 6 , Marta Filizola 3 , Michael A Foley 2 , Barry S Coller 1
ACS Pharmacology & Translational Science Pub Date : 2019-08-16 , DOI: 10.1021/acsptsci.9b00041 Jihong Li 1 , Yoshiyuki Fukase 2 , Yi Shang 3 , Wei Zou 4 , José M Muñoz-Félix 5 , Lorena Buitrago 1 , Johannes van Agthoven 6 , Yixiao Zhang 7 , Ryoma Hara 2 , Yuta Tanaka 2 , Rei Okamoto 2 , Takeshi Yasui 2 , Takashi Nakahata 2 , Toshihiro Imaeda 2 , Kazuyoshi Aso 2 , Yuchen Zhou 3 , Charles Locuson 8 , Dragana Nesic 1 , Mark Duggan 9 , Junichi Takagi 10 , Roger D Vaughan 11 , Thomas Walz 7 , Kairbaan Hodivala-Dilke 5 , Steven L Teitelbaum 4 , M Amin Arnaout 6 , Marta Filizola 3 , Michael A Foley 2 , Barry S Coller 1
Affiliation
|
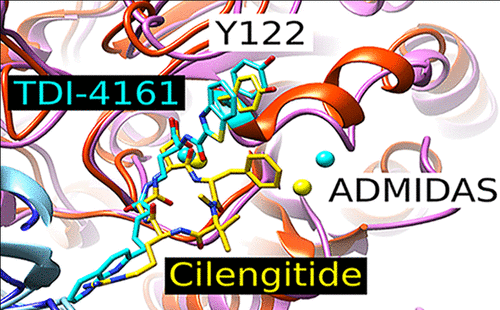
The integrin αVβ3 receptor has been implicated in several important diseases, but no antagonists are approved for human therapy. One possible limitation of current small-molecule antagonists is their ability to induce a major conformational change in the receptor that induces it to adopt a high-affinity ligand-binding state. In response, we used structural inferences from a pure peptide antagonist to design the small-molecule pure antagonists TDI-4161 and TDI-3761. Both compounds inhibit αVβ3-mediated cell adhesion to αVβ3 ligands, but do not induce the conformational change as judged by antibody binding, electron microscopy, X-ray crystallography, and receptor priming studies. Both compounds demonstrated the favorable property of inhibiting bone resorption in vitro, supporting potential value in treating osteoporosis. Neither, however, had the unfavorable property of the αVβ3 antagonist cilengitide of paradoxically enhancing aortic sprout angiogenesis at concentrations below its IC50, which correlates with cilengitide’s enhancement of tumor growth in vivo.
中文翻译:

新型纯 αVβ3 整合素拮抗剂在低浓度下不诱导受体延伸、启动受体或增强血管生成。
整合素 αVβ3 受体与几种重要疾病有关,但没有任何拮抗剂被批准用于人类治疗。当前小分子拮抗剂的一个可能限制是它们能够诱导受体的主要构象变化,从而诱导受体采用高亲和力配体结合状态。作为回应,我们使用纯肽拮抗剂的结构推断来设计小分子纯拮抗剂 TDI-4161 和 TDI-3761。两种化合物均抑制 αVβ3 介导的细胞与 αVβ3 配体的粘附,但不会诱导构象变化,如抗体结合、电子显微镜、X 射线晶体学和受体启动研究所判断的那样。两种化合物都证明了在体外抑制骨吸收的有利特性,支持治疗骨质疏松症的潜在价值。然而,两者都没有 αVβ3 拮抗剂西仑吉肽的不利特性,即在低于其 IC 50的浓度下反常地增强主动脉芽血管生成,这与西仑吉肽在体内肿瘤生长的增强有关。
更新日期:2019-08-18
中文翻译:

新型纯 αVβ3 整合素拮抗剂在低浓度下不诱导受体延伸、启动受体或增强血管生成。
整合素 αVβ3 受体与几种重要疾病有关,但没有任何拮抗剂被批准用于人类治疗。当前小分子拮抗剂的一个可能限制是它们能够诱导受体的主要构象变化,从而诱导受体采用高亲和力配体结合状态。作为回应,我们使用纯肽拮抗剂的结构推断来设计小分子纯拮抗剂 TDI-4161 和 TDI-3761。两种化合物均抑制 αVβ3 介导的细胞与 αVβ3 配体的粘附,但不会诱导构象变化,如抗体结合、电子显微镜、X 射线晶体学和受体启动研究所判断的那样。两种化合物都证明了在体外抑制骨吸收的有利特性,支持治疗骨质疏松症的潜在价值。然而,两者都没有 αVβ3 拮抗剂西仑吉肽的不利特性,即在低于其 IC 50的浓度下反常地增强主动脉芽血管生成,这与西仑吉肽在体内肿瘤生长的增强有关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号