当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enol-imino–Keto-enamine Tautomerism in a Diazepine Derivative: How Decisive Are the Intermolecular Interactions in the Equilibrium?
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2019-08-16 00:00:00 , DOI: 10.1021/acs.joc.9b01533 Mariana Rocha 1 , Diego M. Gil 1 , Gustavo A. Echeverría 2 , Oscar E. Piro 2 , Jorge L. Jios 3 , Sonia E. Ulic 4, 5
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2019-08-16 00:00:00 , DOI: 10.1021/acs.joc.9b01533 Mariana Rocha 1 , Diego M. Gil 1 , Gustavo A. Echeverría 2 , Oscar E. Piro 2 , Jorge L. Jios 3 , Sonia E. Ulic 4, 5
Affiliation
|
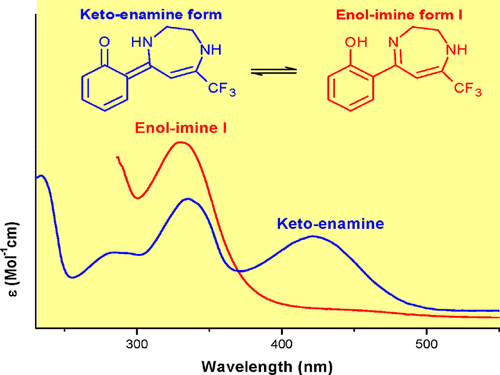
The strange tautomeric equilibrium behavior exhibited by a new o-hydroxyphenyl diazepine derivative when the compound is analyzed both in solution and solid state opens the structural study of the enol-imino–keto-enamine forms and the influence of the intermolecular interactions in their equilibrium. The expected enol-imino form, in which the enol is part of a phenyl system and a strong O–H···N intramolecular hydrogen bond is established, results the most stable tautomer in gas phase (theoretical calculations) and was detected by NMR spectroscopy when the compound was dissolved in aprotic solvents. Nevertheless, the keto-enamine form ,in which the keto group integrates a cyclohexadienone moiety and the aromaticity of the phenol is lost, was the only tautomer in the crystal lattice according to single-crystal X-ray diffraction, vibrational spectroscopy, and diffuse reflectance results. The last form was also found as the main tautomer in UV–vis and NMR spectroscopy when a protic solvent was employed.
中文翻译:

二氮杂pine衍生物中的烯醇-亚氨基-酮-烯胺互变异构:分子间相互作用在平衡中的决定性程度如何?
一个新的o显示出奇怪的互变异构平衡行为-羟苯基二氮杂derivative衍生物在溶液和固体状态下均被分析,这开启了烯醇-亚氨基-酮-烯胺形式的结构研究以及分子间相互作用对其平衡的影响。预期的烯醇-亚氨基形式,其中的烯醇是苯基系统的一部分,并且建立了强的OH-··N分子内氢键,是气相中最稳定的互变异构体(理论计算),并通过NMR进行了检测化合物溶解在非质子传递溶剂中时进行光谱分析。然而,根据单晶X射线衍射,振动光谱和漫反射率,酮基整合了环己二烯酮部分且酚的芳香性丧失的酮烯胺形式是晶格中唯一的互变异构体。结果。
更新日期:2019-08-16
中文翻译:

二氮杂pine衍生物中的烯醇-亚氨基-酮-烯胺互变异构:分子间相互作用在平衡中的决定性程度如何?
一个新的o显示出奇怪的互变异构平衡行为-羟苯基二氮杂derivative衍生物在溶液和固体状态下均被分析,这开启了烯醇-亚氨基-酮-烯胺形式的结构研究以及分子间相互作用对其平衡的影响。预期的烯醇-亚氨基形式,其中的烯醇是苯基系统的一部分,并且建立了强的OH-··N分子内氢键,是气相中最稳定的互变异构体(理论计算),并通过NMR进行了检测化合物溶解在非质子传递溶剂中时进行光谱分析。然而,根据单晶X射线衍射,振动光谱和漫反射率,酮基整合了环己二烯酮部分且酚的芳香性丧失的酮烯胺形式是晶格中唯一的互变异构体。结果。

































 京公网安备 11010802027423号
京公网安备 11010802027423号