当前位置:
X-MOL 学术
›
Mol. Syst. Des. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sequence-dependent self-coacervation in high charge-density polyampholytes
Molecular Systems Design & Engineering ( IF 3.2 ) Pub Date : 2019-08-16 , DOI: 10.1039/c9me00074g Jason J. Madinya 1, 2, 3, 4 , Li-Wei Chang 4, 5, 6, 7 , Sarah L. Perry 4, 5, 6, 7 , Charles E. Sing 1, 2, 3, 4
Molecular Systems Design & Engineering ( IF 3.2 ) Pub Date : 2019-08-16 , DOI: 10.1039/c9me00074g Jason J. Madinya 1, 2, 3, 4 , Li-Wei Chang 4, 5, 6, 7 , Sarah L. Perry 4, 5, 6, 7 , Charles E. Sing 1, 2, 3, 4
Affiliation
|
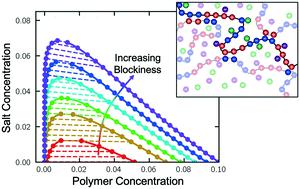
Polyampholytes, which contain both positive and negative charges along the backbone, represent a classical model system for certain types of ‘intrinsically-disordered proteins’ (IDPs). IDPs can possess biological functionality, even in an unfolded state, including the formation of phase-separated regions within a cell; while driven by a number of interactions, electrostatic attractions are thought to be key to forming these structures. This process of electrostatically-driven liquid–liquid phase separation, known as ‘complex coacervation’, can also be observed in simpler polymer or biopolymer systems. In this paper, we use a series of model polyampholytic polypeptides of increasing blockiness, that undergo ‘self-coacervation’ due to charge attraction between polycation and polyanion blocks along the same polymer chain. We show that these polypeptides undergo complex coacervation when sequences have at least 8–12 adjacent like-charges, with increasing blockiness leading to a larger two-phase region. We simultaneously develop a theory built on the transfer-matrix formalism developed by the authors, to show how blockiness increases the strength of electrostatic interactions and subsequently promote phase separation. We explore a tradeoff that emerges due to the presence of ‘charge-pattern interfaces’ where the sequence of polyampholyte charges switches sign, and how these contrast with chain-ends in equivalent homopolyelectrolyte coacervates.
中文翻译:

高电荷密度多两性电解质中依赖序列的自凝聚
沿主链包含正电荷和负电荷的多两性电解质代表了某些类型的“内在无序蛋白”(IDP)的经典模型系统。IDP即使在未折叠状态下也可以具有生物学功能,包括在细胞内形成相分离的区域。尽管受到许多相互作用的驱动,但静电引力被认为是形成这些结构的关键。在更简单的聚合物或生物聚合物系统中,也可以观察到这种静电驱动的液相-液相分离过程,称为“复杂凝聚”。在本文中,我们使用了一系列增加嵌段性的模型多两性多肽,由于聚阳离子和聚阴离子嵌段之间沿着同一条聚合物链的电荷吸引,它们会发生“自凝聚”。我们显示,当序列中至少有8–12个相邻的类似电荷时,这些多肽会经历复杂的凝聚作用,并增加封闭性,从而导致更大的两相区域。我们同时开发了基于作者所建立的转移矩阵形式主义的理论,以显示嵌段性如何增加静电相互作用的强度并随后促进相分离。我们探讨了由于存在“电荷模式界面”而出现的权衡问题,在该模式中,两性电解质电荷的开关序列发生符号变化,以及这些电荷与等效均聚电解质凝聚层中的链端形成对比。我们同时开发了基于作者所建立的转移矩阵形式主义的理论,以显示嵌段性如何增加静电相互作用的强度并随后促进相分离。我们探讨了由于存在“电荷模式界面”而出现的一种折衷,在该模式中,两性电解质电荷的开关序列发生符号变化,以及这些电荷与等效均聚电解质凝聚层中的链端形成对比。我们同时开发了基于作者所建立的转移矩阵形式主义的理论,以显示嵌段性如何增加静电相互作用的强度并随后促进相分离。我们探讨了由于存在“电荷模式界面”而出现的一种折衷,在该模式中,两性电解质电荷的开关序列发生符号变化,以及这些电荷与等效均聚电解质凝聚层中的链端形成对比。
更新日期:2019-08-16
中文翻译:

高电荷密度多两性电解质中依赖序列的自凝聚
沿主链包含正电荷和负电荷的多两性电解质代表了某些类型的“内在无序蛋白”(IDP)的经典模型系统。IDP即使在未折叠状态下也可以具有生物学功能,包括在细胞内形成相分离的区域。尽管受到许多相互作用的驱动,但静电引力被认为是形成这些结构的关键。在更简单的聚合物或生物聚合物系统中,也可以观察到这种静电驱动的液相-液相分离过程,称为“复杂凝聚”。在本文中,我们使用了一系列增加嵌段性的模型多两性多肽,由于聚阳离子和聚阴离子嵌段之间沿着同一条聚合物链的电荷吸引,它们会发生“自凝聚”。我们显示,当序列中至少有8–12个相邻的类似电荷时,这些多肽会经历复杂的凝聚作用,并增加封闭性,从而导致更大的两相区域。我们同时开发了基于作者所建立的转移矩阵形式主义的理论,以显示嵌段性如何增加静电相互作用的强度并随后促进相分离。我们探讨了由于存在“电荷模式界面”而出现的权衡问题,在该模式中,两性电解质电荷的开关序列发生符号变化,以及这些电荷与等效均聚电解质凝聚层中的链端形成对比。我们同时开发了基于作者所建立的转移矩阵形式主义的理论,以显示嵌段性如何增加静电相互作用的强度并随后促进相分离。我们探讨了由于存在“电荷模式界面”而出现的一种折衷,在该模式中,两性电解质电荷的开关序列发生符号变化,以及这些电荷与等效均聚电解质凝聚层中的链端形成对比。我们同时开发了基于作者所建立的转移矩阵形式主义的理论,以显示嵌段性如何增加静电相互作用的强度并随后促进相分离。我们探讨了由于存在“电荷模式界面”而出现的一种折衷,在该模式中,两性电解质电荷的开关序列发生符号变化,以及这些电荷与等效均聚电解质凝聚层中的链端形成对比。











































 京公网安备 11010802027423号
京公网安备 11010802027423号