当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting the N Terminus of eIF4AI for Inhibition of Its Catalytic Recycling.
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2019-08-08 , DOI: 10.1016/j.chembiol.2019.07.010 Chenxiao Jiang 1 , Yegen Tang 2 , Lulu Ding 3 , Renke Tan 4 , Xiaojing Li 4 , Junyan Lu 5 , Jing Jiang 6 , Zhaomeng Cui 4 , Zhewei Tang 4 , Wei Li 7 , Zhangjun Cao 6 , Tilman Schneider-Poetsch 8 , Wei Jiang 4 , Cheng Luo 9 , Yu Ding 3 , Jianwei Liu 2 , Yongjun Dang 4
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2019-08-08 , DOI: 10.1016/j.chembiol.2019.07.010 Chenxiao Jiang 1 , Yegen Tang 2 , Lulu Ding 3 , Renke Tan 4 , Xiaojing Li 4 , Junyan Lu 5 , Jing Jiang 6 , Zhaomeng Cui 4 , Zhewei Tang 4 , Wei Li 7 , Zhangjun Cao 6 , Tilman Schneider-Poetsch 8 , Wei Jiang 4 , Cheng Luo 9 , Yu Ding 3 , Jianwei Liu 2 , Yongjun Dang 4
Affiliation
|
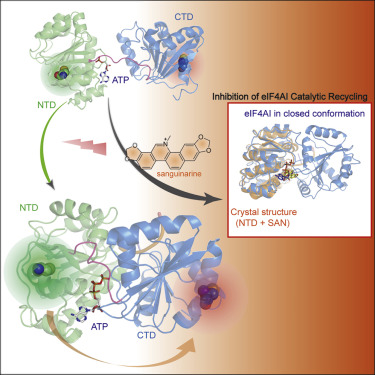
DEAD-box ATP-dependent helicases (DEAH/D) are a family of conserved genes predominantly involved in gene expression regulation and RNA processing. As its prototype, eIF4AI is an essential component of the protein translation initiation complex. Utilizing a screening system based on wild-type eIF4AI and its L243G mutant with a changed linker domain, we discovered an eIF4AI inhibitor, sanguinarine (SAN) and used it to study the catalytic mechanism of eIF4AI. Herein, we describe the crystal structure of the eIF4AI-inhibitor complex and demonstrate that the binding site displays certain specificity, which can provide the basis for drug design to target eIF4AI. We report that except for competitive inhibition SAN's possible mechanism of action involves interference with eIF4AI catalytic cycling process by hindering the formation of the closed conformation of eIF4AI. In addition, our results highlight a new targetable site on eIF4AI and confirm eIF4AI as a viable pharmacological target.
中文翻译:

靶向eIF4AI的N末端来抑制其催化循环。
DEAD-box ATP依赖性解旋酶(DEAH / D)是保守基因家族,主要参与基因表达调控和RNA加工。作为其原型,eIF4AI是蛋白质翻译起始复合体的重要组成部分。利用基于野生型eIF4AI及其具有改变的接头结构域的L243G突变体的筛选系统,我们发现了eIF4AI抑制剂sanguinarine(SAN),并用于研究eIF4AI的催化机理。在这里,我们描述了eIF4AI抑制剂复合物的晶体结构,并证明了结合位点显示出一定的特异性,这可以为靶向eIF4AI的药物设计提供基础。我们报告说,除了竞争抑制外,SAN' 可能的作用机制涉及通过阻止eIF4AI的闭合构象的形成来干扰eIF4AI催化循环过程。此外,我们的结果突出显示了eIF4AI上的一个新的可靶向位点,并确认eIF4AI是可行的药理学靶标。
更新日期:2019-11-09
中文翻译:

靶向eIF4AI的N末端来抑制其催化循环。
DEAD-box ATP依赖性解旋酶(DEAH / D)是保守基因家族,主要参与基因表达调控和RNA加工。作为其原型,eIF4AI是蛋白质翻译起始复合体的重要组成部分。利用基于野生型eIF4AI及其具有改变的接头结构域的L243G突变体的筛选系统,我们发现了eIF4AI抑制剂sanguinarine(SAN),并用于研究eIF4AI的催化机理。在这里,我们描述了eIF4AI抑制剂复合物的晶体结构,并证明了结合位点显示出一定的特异性,这可以为靶向eIF4AI的药物设计提供基础。我们报告说,除了竞争抑制外,SAN' 可能的作用机制涉及通过阻止eIF4AI的闭合构象的形成来干扰eIF4AI催化循环过程。此外,我们的结果突出显示了eIF4AI上的一个新的可靶向位点,并确认eIF4AI是可行的药理学靶标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号