JACC: Heart Failure ( IF 10.3 ) Pub Date : 2019-08-07 , DOI: 10.1016/j.jchf.2019.07.001 Mitchell A Psotka 1 , Mona Fiuzat 2 , Peter E Carson 3 , David P Kao 4 , Jeffrey Cerkvenik 5 , Daniel E Schaber 5 , Patrick Verta 6 , Robert T Kazmierski 7 , Meir Shinnar 7 , Norman Stockbridge 7 , Ellis F Unger 7 , Bram Zuckerman 7 , Javed Butler 8 , G Michael Felker 9 , Marvin A Konstam 10 , JoAnn Lindenfeld 11 , Scott D Solomon 12 , John R Teerlink 13 , Christopher M O'Connor 1 , William T Abraham 14
|
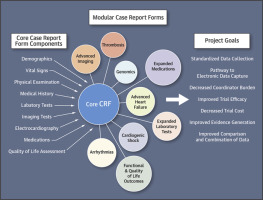
The development of treatments for heart failure (HF) is challenged by burdensome clinical trials. Reducing the need for extensive data collection and increasing opportunities for data compatibility between trials may improve efficiency and reduce resource burden. The Heart Failure Collaboratory (HFC) multi-stakeholder consortium sought to create a lean case report form (CRF) for use in HF clinical trials evaluating cardiac devices. The HFC convened patients, clinicians, clinical researchers, the U.S. Food and Drug Administration (FDA), payers, industry partners, and statisticians to create a consensus core CRF. Eight recent clinical trial CRFs for the treatment of HF from 6 industry partners were analyzed. All CRF elements were systematically reviewed. Those elements deemed critical for data collection in HF clinical trials were used to construct the final, harmonized CRF. The original CRFs included 176 distinct data items covering demographics, vital signs, physical examination, medical history, laboratory and imaging testing, device therapy, medications, functional and quality of life assessment, and outcome events. The resulting, minimally inclusive CRF device contains 75 baseline data items and 6 events, with separate modular additions that can be used depending on the additional detail required for a particular intervention. The consensus electronic form is now freely available for use in clinical trials. Creation of a core CRF is important to improve clinical trial efficiency in HF device development in the United States. This living document intends to reduce clinical trial administrative burden, increase evidence integrity, and improve comparability of clinical data between trials.
中文翻译:

用于心力衰竭治疗开发的“精益”病例报告表的设计。
繁重的临床试验挑战了心力衰竭(HF)治疗方法的开发。减少对大量数据收集的需求,并增加试验之间数据兼容性的机会,可以提高效率并减少资源负担。心力衰竭合作组织(HFC)的多方利益相关者联盟试图创建一个精简病例报告表(CRF),用于评估心脏设备的HF临床试验。HFC召集患者,临床医生,临床研究人员,美国食品和药物管理局(FDA),付款人,行业合作伙伴和统计学家以建立共识性核心CRF。分析了来自6个行业合作伙伴的8个最近的用于治疗HF的临床试验CRF。系统地审查了所有CRF要素。那些在HF临床试验中被认为对数据收集至关重要的要素被用来构建最终的,统一的CRF。原始的CRF包括176个不同的数据项,包括人口统计学,生命体征,体格检查,病史,实验室和影像学检查,设备治疗,药物,生活功能和质量评估以及结果事件。最终得到的,包容性最低的CRF设备包含75个基线数据项和6个事件,可根据特定干预措施所需的其他详细信息使用单独的模块化添加。现在可以免费获得共识电子表格以用于临床试验。在美国,创建核心CRF对提高HF设备开发的临床试验效率非常重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号