Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Programming of Distinct Chemokine-Dependent and -Independent Search Strategies for Th1 and Th2 Cells Optimizes Function at Inflamed Sites.
Immunity ( IF 25.5 ) Pub Date : 2019-08-06 , DOI: 10.1016/j.immuni.2019.06.026 Alison Gaylo-Moynihan 1 , Hen Prizant 1 , Milan Popović 1 , Ninoshka R J Fernandes 2 , Christopher S Anderson 1 , Kevin K Chiou 3 , Hannah Bell 1 , Dillon C Schrock 1 , Justin Schumacher 4 , Tara Capece 1 , Brandon L Walling 1 , David J Topham 1 , Jim Miller 1 , Alan V Smrcka 5 , Minsoo Kim 1 , Angela Hughson 1 , Deborah J Fowell 1
Immunity ( IF 25.5 ) Pub Date : 2019-08-06 , DOI: 10.1016/j.immuni.2019.06.026 Alison Gaylo-Moynihan 1 , Hen Prizant 1 , Milan Popović 1 , Ninoshka R J Fernandes 2 , Christopher S Anderson 1 , Kevin K Chiou 3 , Hannah Bell 1 , Dillon C Schrock 1 , Justin Schumacher 4 , Tara Capece 1 , Brandon L Walling 1 , David J Topham 1 , Jim Miller 1 , Alan V Smrcka 5 , Minsoo Kim 1 , Angela Hughson 1 , Deborah J Fowell 1
Affiliation
|
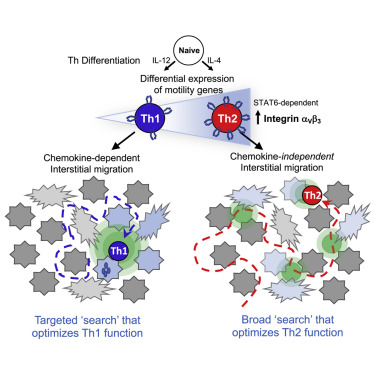
T-helper (Th) cell differentiation drives specialized gene programs that dictate effector T cell function at sites of infection. Here, we have shown Th cell differentiation also imposes discrete motility gene programs that shape Th1 and Th2 cell navigation of the inflamed dermis. Th1 cells scanned a smaller tissue area in a G protein-coupled receptor (GPCR) and chemokine-dependent fashion, while Th2 cells scanned a larger tissue area independent of GPCR signals. Differential chemokine reliance for interstitial migration was linked to STAT6 transcription-factor-dependent programming of integrin αVβ3 expression: Th2 cell differentiation led to high αVβ3 expression relative to Th1 cells. Th1 and Th2 cell modes of motility could be switched simply by manipulating the amount of αVβ3 on the cell surface. Deviating motility modes from those established during differentiation impaired effector function. Thus, programmed expression of αVβ3 tunes effector T cell reliance on environmental cues for optimal exploration of inflamed tissues.
中文翻译:

对 Th1 和 Th2 细胞的不同趋化因子依赖性和独立性搜索策略进行编程可优化炎症部位的功能。
辅助 T 细胞 (Th) 细胞分化驱动专门的基因程序,这些程序决定感染部位的效应 T 细胞功能。在这里,我们发现 Th 细胞分化也会施加离散的运动基因程序,从而塑造发炎真皮的 Th1 和 Th2 细胞导航。Th1 细胞以 G 蛋白偶联受体 (GPCR) 和趋化因子依赖性方式扫描较小的组织区域,而 Th2 细胞则独立于 GPCR 信号扫描较大的组织区域。间质迁移的趋化因子依赖性差异与整合素 αVβ3 表达的 STAT6 转录因子依赖性编程有关:相对于 Th1 细胞,Th2 细胞分化导致 αVβ3 表达较高。Th1 和 Th2 细胞的运动模式可以通过简单地控制细胞表面 αVβ3 的量来切换。偏离分化过程中建立的运动模式会损害效应器功能。因此,αVβ3 的程序化表达调节效应 T 细胞对环境线索的依赖,以实现对发炎组织的最佳探索。
更新日期:2019-08-06
中文翻译:

对 Th1 和 Th2 细胞的不同趋化因子依赖性和独立性搜索策略进行编程可优化炎症部位的功能。
辅助 T 细胞 (Th) 细胞分化驱动专门的基因程序,这些程序决定感染部位的效应 T 细胞功能。在这里,我们发现 Th 细胞分化也会施加离散的运动基因程序,从而塑造发炎真皮的 Th1 和 Th2 细胞导航。Th1 细胞以 G 蛋白偶联受体 (GPCR) 和趋化因子依赖性方式扫描较小的组织区域,而 Th2 细胞则独立于 GPCR 信号扫描较大的组织区域。间质迁移的趋化因子依赖性差异与整合素 αVβ3 表达的 STAT6 转录因子依赖性编程有关:相对于 Th1 细胞,Th2 细胞分化导致 αVβ3 表达较高。Th1 和 Th2 细胞的运动模式可以通过简单地控制细胞表面 αVβ3 的量来切换。偏离分化过程中建立的运动模式会损害效应器功能。因此,αVβ3 的程序化表达调节效应 T 细胞对环境线索的依赖,以实现对发炎组织的最佳探索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号