Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphoproteomics reveals conserved exercise-stimulated signaling and AMPK regulation of store-operated calcium entry.
The EMBO Journal ( IF 9.4 ) Pub Date : 2019-08-05 , DOI: 10.15252/embj.2019102578 Marin E Nelson 1 , Benjamin L Parker 1 , James G Burchfield 1 , Nolan J Hoffman 1 , Elise J Needham 1 , Kristen C Cooke 1 , Timur Naim 2 , Lykke Sylow 3 , Naomi Xy Ling 4 , Deanne Francis 1 , Dougall M Norris 1 , Rima Chaudhuri 1 , Jonathan S Oakhill 4, 5 , Erik A Richter 3 , Gordon S Lynch 2 , Jacqueline Stöckli 1 , David E James 1
The EMBO Journal ( IF 9.4 ) Pub Date : 2019-08-05 , DOI: 10.15252/embj.2019102578 Marin E Nelson 1 , Benjamin L Parker 1 , James G Burchfield 1 , Nolan J Hoffman 1 , Elise J Needham 1 , Kristen C Cooke 1 , Timur Naim 2 , Lykke Sylow 3 , Naomi Xy Ling 4 , Deanne Francis 1 , Dougall M Norris 1 , Rima Chaudhuri 1 , Jonathan S Oakhill 4, 5 , Erik A Richter 3 , Gordon S Lynch 2 , Jacqueline Stöckli 1 , David E James 1
Affiliation
|
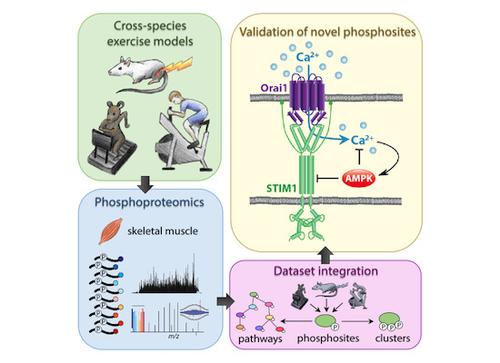
Exercise stimulates cellular and physiological adaptations that are associated with widespread health benefits. To uncover conserved protein phosphorylation events underlying this adaptive response, we performed mass spectrometry-based phosphoproteomic analyses of skeletal muscle from two widely used rodent models: treadmill running in mice and in situ muscle contraction in rats. We overlaid these phosphoproteomic signatures with cycling in humans to identify common cross-species phosphosite responses, as well as unique model-specific regulation. We identified > 22,000 phosphosites, revealing orthologous protein phosphorylation and overlapping signaling pathways regulated by exercise. This included two conserved phosphosites on stromal interaction molecule 1 (STIM1), which we validate as AMPK substrates. Furthermore, we demonstrate that AMPK-mediated phosphorylation of STIM1 negatively regulates store-operated calcium entry, and this is beneficial for exercise in Drosophila. This integrated cross-species resource of exercise-regulated signaling in human, mouse, and rat skeletal muscle has uncovered conserved networks and unraveled crosstalk between AMPK and intracellular calcium flux.
中文翻译:

磷酸蛋白质组学揭示了保守的运动刺激信号传导和AMPK调节储存钙的进入。
运动会刺激与广泛的健康益处相关的细胞和生理适应。为了揭示这种适应性反应背后的保守蛋白磷酸化事件,我们从两种广泛使用的啮齿动物模型中进行了基于质谱的骨骼肌磷酸化蛋白质组学分析:小鼠跑步机和大鼠原位肌肉收缩。我们在人类循环中覆盖了这些磷酸化蛋白质组学特征,以识别常见的跨物种磷酸位点反应以及独特的模型特异性调控。我们确定了> 22,000个磷酸位点,揭示了运动调节的直系同源蛋白质的磷酸化和重叠的信号传导途径。这包括基质相互作用分子1(STIM1)上的两个保守的磷酸位点,我们将其验证为AMPK底物。此外,我们证明AMPK介导的STIM1磷酸化负调控储藏操作的钙进入,这对于在果蝇中锻炼有益。在人,小鼠和大鼠骨骼肌中这种运动调节信号传导的跨物种综合资源,已经发现了AMPK与细胞内钙通量之间的保守网络和未知串扰。
更新日期:2019-12-17
中文翻译:

磷酸蛋白质组学揭示了保守的运动刺激信号传导和AMPK调节储存钙的进入。
运动会刺激与广泛的健康益处相关的细胞和生理适应。为了揭示这种适应性反应背后的保守蛋白磷酸化事件,我们从两种广泛使用的啮齿动物模型中进行了基于质谱的骨骼肌磷酸化蛋白质组学分析:小鼠跑步机和大鼠原位肌肉收缩。我们在人类循环中覆盖了这些磷酸化蛋白质组学特征,以识别常见的跨物种磷酸位点反应以及独特的模型特异性调控。我们确定了> 22,000个磷酸位点,揭示了运动调节的直系同源蛋白质的磷酸化和重叠的信号传导途径。这包括基质相互作用分子1(STIM1)上的两个保守的磷酸位点,我们将其验证为AMPK底物。此外,我们证明AMPK介导的STIM1磷酸化负调控储藏操作的钙进入,这对于在果蝇中锻炼有益。在人,小鼠和大鼠骨骼肌中这种运动调节信号传导的跨物种综合资源,已经发现了AMPK与细胞内钙通量之间的保守网络和未知串扰。











































 京公网安备 11010802027423号
京公网安备 11010802027423号