当前位置:
X-MOL 学术
›
Metabolism
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adaptor protein APPL1 coordinates HDAC3 to modulate brown adipose tissue thermogenesis in mice.
Metabolism ( IF 10.8 ) Pub Date : 2019-08-04 , DOI: 10.1016/j.metabol.2019.153955 Linling Fan 1 , Hongying Ye 1 , Yun Wan 1 , Lang Qin 1 , Lu Zhu 1 , Jing Su 1 , Xiaoming Zhu 1 , Lv Zhang 1 , Qing Miao 1 , Qiongyue Zhang 1 , Zhaoyun Zhang 1 , Aimin Xu 2 , Yiming Li 1 , Xi Li 3 , Yi Wang 1
Metabolism ( IF 10.8 ) Pub Date : 2019-08-04 , DOI: 10.1016/j.metabol.2019.153955 Linling Fan 1 , Hongying Ye 1 , Yun Wan 1 , Lang Qin 1 , Lu Zhu 1 , Jing Su 1 , Xiaoming Zhu 1 , Lv Zhang 1 , Qing Miao 1 , Qiongyue Zhang 1 , Zhaoyun Zhang 1 , Aimin Xu 2 , Yiming Li 1 , Xi Li 3 , Yi Wang 1
Affiliation
|
OBJECTIVES
The activation of brown adipose tissue (BAT) is considered as a promising therapeutic target for obesity. APPL1 (Adaptor protein containing the Pleckstrin homology domain, Phosphotyrosine binding domain and Leucine zipper motif) is an intracellular adaptor protein and its genetic variation is correlated with BMI and body fat distribution in diabetic patients. However, little is known about the roles of APPL1 in BAT thermogenesis.
MATERIALS/METHODS
In this study, adipose tissue specific knockout (ASKO) mice were generated to evaluate APPL1's role in BAT thermogenesis in vivo, and possible signaling pathways were further explored in cultured brown adipocytes.
RESULTS
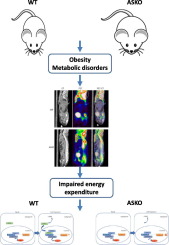
After high fat diet challenge, APPL1 ASKO mice developed more severe obesity, glucose intolerance and insulin resistance compared with control mice. Metabolic cage study showed that APPL1 deficiency impaired energy expenditure and adaptive thermogenesis in ASKO mice. PET-CT analysis showed decreased standardized uptake value (SUV) in the inter-scapular region which indicated impaired BAT activity in ASKO mice. Further study showed deletion of APPL1 attenuated brown fat specific gene expression, such as UCP1 and PGC1α in both BAT and brown adipocytes. In cultured brown adipocytes, upon cAMP stimulation, APPL1 shuttled from cytosol to nuclei. Co-IP and ChIP study showed that APPL1 could directly interact with histone deacetylase 3 (HDAC3) to mediate chromatin remodeling and UCP1 gene expression.
CONCLUSIONS
Our data demonstrated the essential role of APPL1 in regulating brown adipocytes thermogenesis via interaction with HDAC3, which may have potential therapeutic implications for treatment of obesity.
中文翻译:

衔接蛋白APPL1协调HDAC3以调节小鼠棕色脂肪组织的生热作用。
目的棕色脂肪组织(BAT)的激活被认为是肥胖的有希望的治疗靶标。APPL1(含有Pleckstrin同源结构域,磷酸酪氨酸结合结构域和亮氨酸拉链基序的衔接蛋白)是一种细胞内衔接蛋白,其遗传变异与糖尿病患者的BMI和体脂分布有关。但是,关于BAT生热中APPL1的作用知之甚少。材料/方法在这项研究中,生成了脂肪组织特异性基因敲除(ASKO)小鼠,以评估APPL1在体内BAT生热中的作用,并在培养的棕色脂肪细胞中进一步探索了可能的信号通路。结果在高脂饮食挑战后,APPL1 ASKO小鼠比对照组小鼠出现了更严重的肥胖,葡萄糖耐量和胰岛素抵抗。代谢笼研究表明,APPL1缺乏会损害ASKO小鼠的能量消耗和适应性生热作用。PET-CT分析显示肩s间区域的标准摄取值(SUV)降低,这表明ASKO小鼠的BAT活性受损。进一步的研究表明,在BAT和棕色脂肪细胞中,APPL1的缺失会减弱棕色脂肪特异性基因的表达,例如UCP1和PGC1α。在培养的棕色脂肪细胞中,在cAMP刺激下,APPL1从细胞质穿梭到细胞核。Co-IP和ChIP研究表明,APPL1可以直接与组蛋白脱乙酰基酶3(HDAC3)相互作用,介导染色质重塑和UCP1基因表达。结论我们的数据表明APPL1通过与HDAC3的相互作用在调节棕色脂肪细胞的生热中起着至关重要的作用,
更新日期:2019-08-04
中文翻译:

衔接蛋白APPL1协调HDAC3以调节小鼠棕色脂肪组织的生热作用。
目的棕色脂肪组织(BAT)的激活被认为是肥胖的有希望的治疗靶标。APPL1(含有Pleckstrin同源结构域,磷酸酪氨酸结合结构域和亮氨酸拉链基序的衔接蛋白)是一种细胞内衔接蛋白,其遗传变异与糖尿病患者的BMI和体脂分布有关。但是,关于BAT生热中APPL1的作用知之甚少。材料/方法在这项研究中,生成了脂肪组织特异性基因敲除(ASKO)小鼠,以评估APPL1在体内BAT生热中的作用,并在培养的棕色脂肪细胞中进一步探索了可能的信号通路。结果在高脂饮食挑战后,APPL1 ASKO小鼠比对照组小鼠出现了更严重的肥胖,葡萄糖耐量和胰岛素抵抗。代谢笼研究表明,APPL1缺乏会损害ASKO小鼠的能量消耗和适应性生热作用。PET-CT分析显示肩s间区域的标准摄取值(SUV)降低,这表明ASKO小鼠的BAT活性受损。进一步的研究表明,在BAT和棕色脂肪细胞中,APPL1的缺失会减弱棕色脂肪特异性基因的表达,例如UCP1和PGC1α。在培养的棕色脂肪细胞中,在cAMP刺激下,APPL1从细胞质穿梭到细胞核。Co-IP和ChIP研究表明,APPL1可以直接与组蛋白脱乙酰基酶3(HDAC3)相互作用,介导染色质重塑和UCP1基因表达。结论我们的数据表明APPL1通过与HDAC3的相互作用在调节棕色脂肪细胞的生热中起着至关重要的作用,











































 京公网安备 11010802027423号
京公网安备 11010802027423号