Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ursodeoxycholic acid versus placebo in women with intrahepatic cholestasis of pregnancy (PITCHES): a randomised controlled trial.
The Lancet ( IF 98.4 ) Pub Date : 2019-08-01 , DOI: 10.1016/s0140-6736(19)31270-x Lucy C Chappell 1 , Jennifer L Bell 2 , Anne Smith 2 , Louise Linsell 2 , Edmund Juszczak 2 , Peter H Dixon 1 , Jenny Chambers 3 , Rachael Hunter 4 , Jon Dorling 5 , Catherine Williamson 1 , Jim G Thornton 6 ,
The Lancet ( IF 98.4 ) Pub Date : 2019-08-01 , DOI: 10.1016/s0140-6736(19)31270-x Lucy C Chappell 1 , Jennifer L Bell 2 , Anne Smith 2 , Louise Linsell 2 , Edmund Juszczak 2 , Peter H Dixon 1 , Jenny Chambers 3 , Rachael Hunter 4 , Jon Dorling 5 , Catherine Williamson 1 , Jim G Thornton 6 ,
Affiliation
|
BACKGROUND
Intrahepatic cholestasis of pregnancy, characterised by maternal pruritus and increased serum bile acid concentrations, is associated with increased rates of stillbirth, preterm birth, and neonatal unit admission. Ursodeoxycholic acid is widely used as a treatment without an adequate evidence base. We aimed to evaluate whether ursodeoxycholic acid reduces adverse perinatal outcomes in women with intrahepatic cholestasis of pregnancy.
METHODS
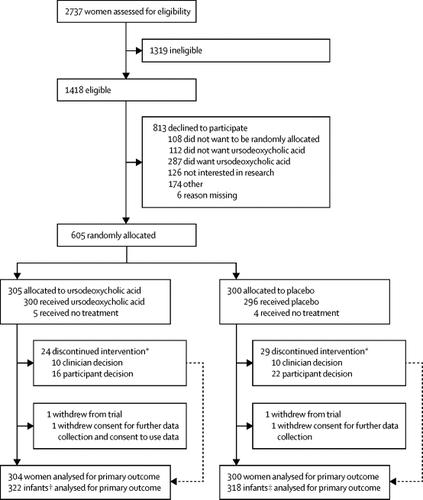
We did a double-blind, multicentre, randomised placebo-controlled trial at 33 hospital maternity units in England and Wales. We recruited women with intrahepatic cholestasis of pregnancy, who were aged 18 years or older and with a gestational age between 20 weeks and 40 weeks and 6 days, with a singleton or twin pregnancy and no known lethal fetal anomaly. Participants were randomly assigned 1:1 to ursodeoxycholic acid or placebo, given as two oral tablets a day at an equivalent dose of 500 mg twice a day. The dose could be increased or decreased at the clinician's discretion, to a maximum of four tablets and a minimum of one tablet a day. We recommended that treatment should be continued from enrolment until the infant's birth. The primary outcome was a composite of perinatal death (in-utero fetal death after randomisation or known neonatal death up to 7 days after birth), preterm delivery (<37 weeks' gestation), or neonatal unit admission for at least 4 h (from birth until hospital discharge). Each infant was counted once within this composite. All analyses were done according to the intention-to-treat principle. The trial was prospectively registered with the ISRCTN registry, number 91918806.
FINDINGS
Between Dec 23, 2015, and Aug 7, 2018, 605 women were enrolled and randomly allocated to receive ursodeoxycholic acid (n=305) or placebo (n=300). The primary outcome analysis included 304 women and 322 infants in the ursodeoxycholic acid group, and 300 women and 318 infants in the placebo group (consent to use data was withdrawn for 1 woman and 2 infants). The primary composite outcome occurred in 74 (23%) of 322 infants in the ursodeoxycholic acid group and 85 (27%) of 318 infants in the placebo group (adjusted risk ratio 0·85 [95% CI 0·62-1·15]). Two serious adverse events were reported in the ursodeoxycholic acid group and six serious adverse events were reported in the placebo group; no serious adverse events were regarded as being related to treatment.
INTERPRETATION
Treatment with ursodeoxycholic acid does not reduce adverse perinatal outcomes in women with intrahepatic cholestasis of pregnancy. Therefore, its routine use for this condition should be reconsidered.
FUNDING
National Institute for Health Research Efficacy and Mechanism Evaluation Programme.
中文翻译:

妊娠肝内胆汁淤积症(PITCHES)妇女的熊去氧胆酸与安慰剂比较:一项随机对照试验。
背景技术妊娠肝内胆汁淤积症的特征在于母亲瘙痒和血清胆汁酸浓度增加,与死产,早产和新生儿入院率增加有关。熊去氧胆酸被广泛用作没有足够证据基础的治疗方法。我们旨在评估熊去氧胆酸是否可降低妊娠肝内胆汁淤积症妇女的围产期不良后果。方法我们在英格兰和威尔士的33个妇产科医院进行了一项双盲,多中心,随机安慰剂对照试验。我们招募了患有肝内胆汁淤积的孕妇,年龄在18岁或以上,胎龄在20周至40周至6天之间,单胎或双胎妊娠,并且没有致命的胎儿异常。参与者被随机分配为1:1至熊去氧胆酸或安慰剂,每天两次口服,剂量相等,每日两次,每次500 mg。剂量可以根据临床医生的决定增加或减少,每天最多4片,最少1片。我们建议治疗应从入选开始一直持续到婴儿出生为止。主要结局是围产期死亡(随机分组后宫内胎儿死亡或出生后7天以内的已知新生儿死亡),早产(妊娠<37周)或新生儿入院至少4 h(自出生直到出院)。在该复合物中,每个婴儿均被计数一次。所有分析均根据意向性治疗原则进行。该试验已在ISRCTN注册中心进行了预期注册,注册号为91918806。调查结果:2015年和2018年8月7日,招募了605名妇女并随机分配他们接受熊去氧胆酸(n = 305)或安慰剂(n = 300)。主要结果分析包括熊去氧胆酸组的304名妇女和322名婴儿,安慰剂组的300名妇女和318婴儿(同意撤消1名妇女和2名婴儿的使用数据)。熊去氧胆酸组的322例婴儿中有74例(23%)发生了初步复合结局,安慰剂组318例的318例中有85例(27%)(校正后的危险比0·85 [95%CI 0·62-1·15] ])。熊去氧胆酸组报告了2个严重不良事件,安慰剂组报告了6个严重不良事件。没有严重的不良事件被认为与治疗有关。解释妊娠肝内胆汁淤积的妇女使用熊去氧胆酸治疗并不能减少不良的围产期结局。因此,应重新考虑其在这种情况下的常规使用。资助国立卫生研究院功效和机制评估计划。
更新日期:2019-09-06
中文翻译:

妊娠肝内胆汁淤积症(PITCHES)妇女的熊去氧胆酸与安慰剂比较:一项随机对照试验。
背景技术妊娠肝内胆汁淤积症的特征在于母亲瘙痒和血清胆汁酸浓度增加,与死产,早产和新生儿入院率增加有关。熊去氧胆酸被广泛用作没有足够证据基础的治疗方法。我们旨在评估熊去氧胆酸是否可降低妊娠肝内胆汁淤积症妇女的围产期不良后果。方法我们在英格兰和威尔士的33个妇产科医院进行了一项双盲,多中心,随机安慰剂对照试验。我们招募了患有肝内胆汁淤积的孕妇,年龄在18岁或以上,胎龄在20周至40周至6天之间,单胎或双胎妊娠,并且没有致命的胎儿异常。参与者被随机分配为1:1至熊去氧胆酸或安慰剂,每天两次口服,剂量相等,每日两次,每次500 mg。剂量可以根据临床医生的决定增加或减少,每天最多4片,最少1片。我们建议治疗应从入选开始一直持续到婴儿出生为止。主要结局是围产期死亡(随机分组后宫内胎儿死亡或出生后7天以内的已知新生儿死亡),早产(妊娠<37周)或新生儿入院至少4 h(自出生直到出院)。在该复合物中,每个婴儿均被计数一次。所有分析均根据意向性治疗原则进行。该试验已在ISRCTN注册中心进行了预期注册,注册号为91918806。调查结果:2015年和2018年8月7日,招募了605名妇女并随机分配他们接受熊去氧胆酸(n = 305)或安慰剂(n = 300)。主要结果分析包括熊去氧胆酸组的304名妇女和322名婴儿,安慰剂组的300名妇女和318婴儿(同意撤消1名妇女和2名婴儿的使用数据)。熊去氧胆酸组的322例婴儿中有74例(23%)发生了初步复合结局,安慰剂组318例的318例中有85例(27%)(校正后的危险比0·85 [95%CI 0·62-1·15] ])。熊去氧胆酸组报告了2个严重不良事件,安慰剂组报告了6个严重不良事件。没有严重的不良事件被认为与治疗有关。解释妊娠肝内胆汁淤积的妇女使用熊去氧胆酸治疗并不能减少不良的围产期结局。因此,应重新考虑其在这种情况下的常规使用。资助国立卫生研究院功效和机制评估计划。











































 京公网安备 11010802027423号
京公网安备 11010802027423号