当前位置:
X-MOL 学术
›
Mol. Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pharmacological and Genetic Inhibition of Caveolin-1 Promotes Epithelialization and Wound Closure.
Molecular Therapy ( IF 12.4 ) Pub Date : 2019-07-30 , DOI: 10.1016/j.ymthe.2019.07.016 Ivan Jozic 1 , Andrew P Sawaya 1 , Irena Pastar 1 , Cheyanne R Head 1 , Lulu L Wong 1 , George D Glinos 1 , Tongyu Cao Wikramanayake 1 , Harold Brem 2 , Robert S Kirsner 1 , Marjana Tomic-Canic 3
Molecular Therapy ( IF 12.4 ) Pub Date : 2019-07-30 , DOI: 10.1016/j.ymthe.2019.07.016 Ivan Jozic 1 , Andrew P Sawaya 1 , Irena Pastar 1 , Cheyanne R Head 1 , Lulu L Wong 1 , George D Glinos 1 , Tongyu Cao Wikramanayake 1 , Harold Brem 2 , Robert S Kirsner 1 , Marjana Tomic-Canic 3
Affiliation
|
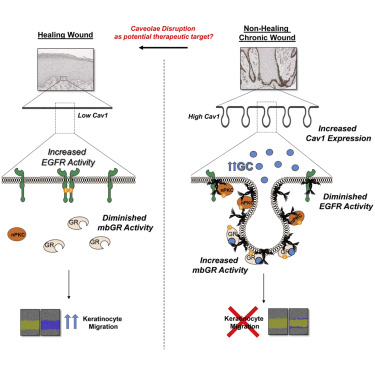
Chronic wounds-including diabetic foot ulcers, venous leg ulcers, and pressure ulcers-represent a major health problem that demands an urgent solution and new therapies. Despite major burden to patients, health care professionals, and health care systems worldwide, there are no efficacious therapies approved for treatment of chronic wounds. One of the major obstacles in achieving wound closure in patients is the lack of epithelial migration. Here, we used multiple pre-clinical wound models to show that Caveolin-1 (Cav1) impedes healing and that targeting Cav1 accelerates wound closure. We found that Cav1 expression is significantly upregulated in wound edge biopsies of patients with non-healing wounds, confirming its healing-inhibitory role. Conversely, Cav1 was absent from the migrating epithelium and is downregulated in acutely healing wounds. Specifically, Cav1 interacted with membranous glucocorticoid receptor (mbGR) and epidermal growth factor receptor (EGFR) in a glucocorticoid-dependent manner to inhibit cutaneous healing. However, pharmacological disruption of caveolae by MβCD or CRISPR/Cas9-mediated Cav1 knockdown resulted in disruption of Cav1-mbGR and Cav1-EGFR complexes and promoted epithelialization and wound healing. Our data reveal a novel mechanism of inhibition of epithelialization and wound closure, providing a rationale for pharmacological targeting of Cav1 as potential therapy for patients with non-healing chronic wounds.
中文翻译:

Caveolin-1 的药理学和遗传抑制促进上皮化和伤口闭合。
慢性伤口——包括糖尿病足溃疡、静脉性腿部溃疡和压疮——代表了一个需要紧急解决方案和新疗法的主要健康问题。尽管全世界的患者、医疗保健专业人员和医疗保健系统都承受着沉重的负担,但没有批准用于治疗慢性伤口的有效疗法。实现患者伤口闭合的主要障碍之一是缺乏上皮迁移。在这里,我们使用多个临床前伤口模型来证明 Caveolin-1 (Cav1) 会阻碍愈合,而靶向 Cav1 会加速伤口闭合。我们发现 Cav1 表达在未愈合伤口患者的伤口边缘活检中显着上调,证实了其抑制愈合作用。相反,迁移的上皮细胞中不存在 Cav1,并且在急性愈合伤口中被下调。具体来说,Cav1 以糖皮质激素依赖的方式与膜性糖皮质激素受体 (mbGR) 和表皮生长因子受体 (EGFR) 相互作用,从而抑制皮肤愈合。然而,MβCD 或 CRISPR/Cas9 介导的 Cav1 敲低对小窝的药理学破坏导致 Cav1-mbGR 和 Cav1-EGFR 复合物的破坏,并促进上皮化和伤口愈合。我们的数据揭示了一种抑制上皮化和伤口闭合的新机制,为 Cav1 的药理学靶向治疗作为慢性伤口不愈合患者的潜在疗法提供了理论依据。MβCD 或 CRISPR/Cas9 介导的 Cav1 敲低对小窝的药理学破坏导致 Cav1-mbGR 和 Cav1-EGFR 复合物的破坏,并促进上皮化和伤口愈合。我们的数据揭示了一种抑制上皮化和伤口闭合的新机制,为 Cav1 的药理学靶向治疗作为慢性伤口不愈合患者的潜在疗法提供了理论基础。MβCD 或 CRISPR/Cas9 介导的 Cav1 敲低对小窝的药理学破坏导致 Cav1-mbGR 和 Cav1-EGFR 复合物的破坏,并促进上皮化和伤口愈合。我们的数据揭示了一种抑制上皮化和伤口闭合的新机制,为 Cav1 的药理学靶向治疗作为慢性伤口不愈合患者的潜在疗法提供了理论依据。
更新日期:2019-07-30
中文翻译:

Caveolin-1 的药理学和遗传抑制促进上皮化和伤口闭合。
慢性伤口——包括糖尿病足溃疡、静脉性腿部溃疡和压疮——代表了一个需要紧急解决方案和新疗法的主要健康问题。尽管全世界的患者、医疗保健专业人员和医疗保健系统都承受着沉重的负担,但没有批准用于治疗慢性伤口的有效疗法。实现患者伤口闭合的主要障碍之一是缺乏上皮迁移。在这里,我们使用多个临床前伤口模型来证明 Caveolin-1 (Cav1) 会阻碍愈合,而靶向 Cav1 会加速伤口闭合。我们发现 Cav1 表达在未愈合伤口患者的伤口边缘活检中显着上调,证实了其抑制愈合作用。相反,迁移的上皮细胞中不存在 Cav1,并且在急性愈合伤口中被下调。具体来说,Cav1 以糖皮质激素依赖的方式与膜性糖皮质激素受体 (mbGR) 和表皮生长因子受体 (EGFR) 相互作用,从而抑制皮肤愈合。然而,MβCD 或 CRISPR/Cas9 介导的 Cav1 敲低对小窝的药理学破坏导致 Cav1-mbGR 和 Cav1-EGFR 复合物的破坏,并促进上皮化和伤口愈合。我们的数据揭示了一种抑制上皮化和伤口闭合的新机制,为 Cav1 的药理学靶向治疗作为慢性伤口不愈合患者的潜在疗法提供了理论依据。MβCD 或 CRISPR/Cas9 介导的 Cav1 敲低对小窝的药理学破坏导致 Cav1-mbGR 和 Cav1-EGFR 复合物的破坏,并促进上皮化和伤口愈合。我们的数据揭示了一种抑制上皮化和伤口闭合的新机制,为 Cav1 的药理学靶向治疗作为慢性伤口不愈合患者的潜在疗法提供了理论基础。MβCD 或 CRISPR/Cas9 介导的 Cav1 敲低对小窝的药理学破坏导致 Cav1-mbGR 和 Cav1-EGFR 复合物的破坏,并促进上皮化和伤口愈合。我们的数据揭示了一种抑制上皮化和伤口闭合的新机制,为 Cav1 的药理学靶向治疗作为慢性伤口不愈合患者的潜在疗法提供了理论依据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号