当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergistic Inhibition of Kinase Pathways Overcomes Resistance of Colorectal Cancer Spheroids to Cyclic Targeted Therapies.
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2019-07-29 , DOI: 10.1021/acsptsci.9b00042 Pradip Shahi Thakuri 1 , Megha Gupta 2 , Ramila Joshi 1 , Sunil Singh 1 , Hossein Tavana 1
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2019-07-29 , DOI: 10.1021/acsptsci.9b00042 Pradip Shahi Thakuri 1 , Megha Gupta 2 , Ramila Joshi 1 , Sunil Singh 1 , Hossein Tavana 1
Affiliation
|
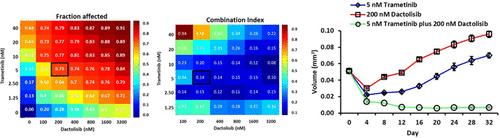
Cancer cells often adapt to single-agent treatments with chemotherapeutics. Activation of alternative survival pathways is a major mechanism of drug resistance. A potential approach to block this feedback signaling is using combination treatments of a pair of drugs, although toxicity has been a limiting factor. Preclinical tumor models to identify mechanisms of drug resistance and determine low but effective combination doses are critical to effectively suppress tumor growth with reduced toxicity to patients. Using our aqueous two-phase system microtechnology, we developed colorectal tumor spheroids in high-throughput and evaluated resistance of cancer cells to three mitogen-activated protein kinase inhibitors (MAPKi) in long-term cyclic treatments. Our quantitative analysis showed that the efficacy of MAPKi significantly reduced over time, leading to an increase in proliferation of HCT116 colorectal cancer cells and growth of spheroids. We established that resistance was due to feedback activation of PI3K/AKT/mTOR pathway. Using high-throughput, dose-dependent combinations of each MAPKi and a PI3K/mTOR inhibitor, we identified low-dose, synergistic combinations that blocked resistance to MAPKi and effectively suppressed the growth of colorectal tumor spheroids in long-term treatments. Our approach to study drug resistance offers the potential to determine high priority treatments to test in animal models.
中文翻译:

激酶途径的协同抑制作用克服了结直肠癌球体对循环靶向疗法的耐药性。
癌细胞通常适用于化学疗法的单药治疗。替代生存途径的激活是耐药性的主要机制。尽管毒性一直是限制因素,但一种潜在的阻断这种反馈信号的方法是使用两种药物的联合治疗。临床前肿瘤模型可识别耐药机制并确定低剂量但有效的联合剂量,对于有效抑制肿瘤生长并降低对患者的毒性至关重要。使用我们的水性两相系统微技术,我们以高通量开发了结直肠肿瘤球体,并在长期循环治疗中评估了癌细胞对三种促分裂原活化蛋白激酶抑制剂(MAPKi)的耐药性。我们的定量分析表明,随着时间的流逝,MAPKi的功效显着降低,导致HCT116大肠癌细胞的增殖增加和球状体的生长。我们确定抗药性是由于PI3K / AKT / mTOR途径的反馈激活所致。使用每种MAPKi和PI3K / mTOR抑制剂的高通量剂量依赖性组合,我们确定了低剂量协同组合,这些组合可阻断MAPKi的耐药性并在长期治疗中有效抑制结直肠肿瘤球的生长。我们研究耐药性的方法提供了确定高优先级治疗方法以在动物模型中进行测试的潜力。我们确定了低剂量,协同作用的组合,这些组合在长期治疗中可阻断MAPKi的耐药性并有效抑制结直肠肿瘤球体的生长。我们研究耐药性的方法提供了确定高优先级治疗方法以在动物模型中进行测试的潜力。我们确定了低剂量,协同作用的组合,这些组合在长期治疗中可阻断MAPKi的耐药性并有效抑制结直肠肿瘤球体的生长。我们研究耐药性的方法提供了确定高优先级治疗方法以在动物模型中进行测试的潜力。
更新日期:2019-07-29
中文翻译:

激酶途径的协同抑制作用克服了结直肠癌球体对循环靶向疗法的耐药性。
癌细胞通常适用于化学疗法的单药治疗。替代生存途径的激活是耐药性的主要机制。尽管毒性一直是限制因素,但一种潜在的阻断这种反馈信号的方法是使用两种药物的联合治疗。临床前肿瘤模型可识别耐药机制并确定低剂量但有效的联合剂量,对于有效抑制肿瘤生长并降低对患者的毒性至关重要。使用我们的水性两相系统微技术,我们以高通量开发了结直肠肿瘤球体,并在长期循环治疗中评估了癌细胞对三种促分裂原活化蛋白激酶抑制剂(MAPKi)的耐药性。我们的定量分析表明,随着时间的流逝,MAPKi的功效显着降低,导致HCT116大肠癌细胞的增殖增加和球状体的生长。我们确定抗药性是由于PI3K / AKT / mTOR途径的反馈激活所致。使用每种MAPKi和PI3K / mTOR抑制剂的高通量剂量依赖性组合,我们确定了低剂量协同组合,这些组合可阻断MAPKi的耐药性并在长期治疗中有效抑制结直肠肿瘤球的生长。我们研究耐药性的方法提供了确定高优先级治疗方法以在动物模型中进行测试的潜力。我们确定了低剂量,协同作用的组合,这些组合在长期治疗中可阻断MAPKi的耐药性并有效抑制结直肠肿瘤球体的生长。我们研究耐药性的方法提供了确定高优先级治疗方法以在动物模型中进行测试的潜力。我们确定了低剂量,协同作用的组合,这些组合在长期治疗中可阻断MAPKi的耐药性并有效抑制结直肠肿瘤球体的生长。我们研究耐药性的方法提供了确定高优先级治疗方法以在动物模型中进行测试的潜力。









































 京公网安备 11010802027423号
京公网安备 11010802027423号