当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study on Preferential Solvation of Water by Electrochemical Method
Electroanalysis ( IF 2.7 ) Pub Date : 2019-07-25 , DOI: 10.1002/elan.201900243 Chunxia Yan 1 , Ximing Huang 1 , Jingchao Chen 1 , Haixia Guo 1 , Huibo Shao 1
Electroanalysis ( IF 2.7 ) Pub Date : 2019-07-25 , DOI: 10.1002/elan.201900243 Chunxia Yan 1 , Ximing Huang 1 , Jingchao Chen 1 , Haixia Guo 1 , Huibo Shao 1
Affiliation
|
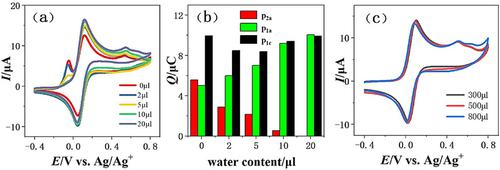
The preferential solvation of water plays an important role in ferrocene research which is a subject of current interest. Voltammetric investigations were carried out for Au electrode in acetonitrile/water, showing preferential solvation of water. In our work, the preferential solvation of water in acetonitrile/water was studied by electrochemical methods including cyclic volitammetry, electrochemical impedance spectra and double‐step chronoamperometry. Ferrocenemethanol (FcCH2OH) molecules as a solute spontaneously adsorb on the electrode surface in anhydrous acetonitrile, resulting from acetonitrile molecules tend to form an acetonitrile solvent layer on the surface of the electrode and acetonitrile solvent layer has a lower energy barrier than the aqueous solvent layer, which has been obtained by modeling solvation. The solvent strongly influences electrochemical behavior of solute. Once there is an amount of water in acetonitrile solvent, FcCH2OH that adsorbed on the electrode surface desorb. This is because water preferentially solvate with FcCH2OH in term of intermolecular forces between solvent and solute. Moreover, hydrogen bond between water molecules and FcCH2OH molecules is stronger than dipole‐dipole interaction between acetonitrile molecules and FcCH2OH molecules in solvation effect. Through electrochemical behavior of FcCH2OH changing, preferential solvation of water is analyzed by electrochemical methods.
中文翻译:

电化学法优先处理水的研究
水的优先溶剂化在二茂铁研究中起着重要的作用,这是当前引起关注的主题。在乙腈/水中对金电极进行了伏安研究,显示了优先的水溶剂化作用。在我们的工作中,通过循环伏安法,电化学阻抗谱和双步计时安培法等电化学方法研究了水在乙腈/水中的优先溶剂化。二茂铁甲醇(FcCH 2OH)分子作为溶质自发地吸附在无水乙腈的电极表面上,由乙腈分子导致倾向于在电极表面上形成乙腈溶剂层,且乙腈溶剂层的能垒比水性溶剂层低通过建模溶剂化获得。溶剂强烈影响溶质的电化学行为。一旦在乙腈溶剂中有一定量的水,吸附在电极表面的FcCH 2 OH就会解吸。这是因为就溶剂和溶质之间的分子间力而言,水优先与FcCH 2 OH溶剂化。此外,水分子与FcCH 2之间的氢键OH分子在溶剂化作用方面比乙腈分子与FcCH 2 OH分子之间的偶极-偶极相互作用更强。通过改变FcCH 2 OH的电化学行为,通过电化学方法分析了水的优先溶剂化。
更新日期:2019-11-18
中文翻译:

电化学法优先处理水的研究
水的优先溶剂化在二茂铁研究中起着重要的作用,这是当前引起关注的主题。在乙腈/水中对金电极进行了伏安研究,显示了优先的水溶剂化作用。在我们的工作中,通过循环伏安法,电化学阻抗谱和双步计时安培法等电化学方法研究了水在乙腈/水中的优先溶剂化。二茂铁甲醇(FcCH 2OH)分子作为溶质自发地吸附在无水乙腈的电极表面上,由乙腈分子导致倾向于在电极表面上形成乙腈溶剂层,且乙腈溶剂层的能垒比水性溶剂层低通过建模溶剂化获得。溶剂强烈影响溶质的电化学行为。一旦在乙腈溶剂中有一定量的水,吸附在电极表面的FcCH 2 OH就会解吸。这是因为就溶剂和溶质之间的分子间力而言,水优先与FcCH 2 OH溶剂化。此外,水分子与FcCH 2之间的氢键OH分子在溶剂化作用方面比乙腈分子与FcCH 2 OH分子之间的偶极-偶极相互作用更强。通过改变FcCH 2 OH的电化学行为,通过电化学方法分析了水的优先溶剂化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号