当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selenium-Catalyzed Carbonylative Synthesis of 3,4-Dihydroquinazolin-2(1H)-one Derivatives with TFBen as the CO Source.
ACS Combinatorial Science Pub Date : 2019-07-23 , DOI: 10.1021/acscombsci.9b00090 Rong Zhou 1 , Xinxin Qi 1 , Xiao-Feng Wu 1, 2
ACS Combinatorial Science Pub Date : 2019-07-23 , DOI: 10.1021/acscombsci.9b00090 Rong Zhou 1 , Xinxin Qi 1 , Xiao-Feng Wu 1, 2
Affiliation
|
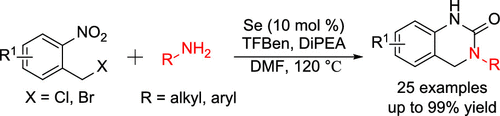
An efficient and general carbonylative procedure for the synthesis of 3,4-dihydroquinazolin-2(1H)-one from 1-(halomethyl)-2-nitrobenzenes and aryl/alkyl amines have been explored. In this approach, to avoid of using toxic CO gas, a solid and stable CO precursor, TFBen (benzene-1,3,5-triyl triformate), was utilized. With elemental selenium as the catalyst, a variety of aryl/alkyl amines has been tolerated well to afford the corresponding 3,4-dihydroquinazolin-2(1H)-one products in moderate to excellent yields under mild reaction condition.
中文翻译:

以TFBen为CO源的硒催化3,4-二氢喹唑啉-2(1H)-one衍生物的羰基化合成。
已经研究了由1-(卤甲基)-2-硝基苯和芳基/烷基胺合成3,4-二氢喹唑啉-2(1H)-一的有效且通用的羰基化方法。在这种方法中,为避免使用有毒的CO气体,使用了固态且稳定的CO前驱物TFBen(苯甲酸1,3,5-5-三甲酸三乙酯)。以元素硒为催化剂,可以很好地耐受各种芳基/烷基胺,从而在温和的反应条件下以中等至极好的收率得到相应的3,4-二氢喹唑啉-2(1H)-一产物。
更新日期:2019-07-18
中文翻译:

以TFBen为CO源的硒催化3,4-二氢喹唑啉-2(1H)-one衍生物的羰基化合成。
已经研究了由1-(卤甲基)-2-硝基苯和芳基/烷基胺合成3,4-二氢喹唑啉-2(1H)-一的有效且通用的羰基化方法。在这种方法中,为避免使用有毒的CO气体,使用了固态且稳定的CO前驱物TFBen(苯甲酸1,3,5-5-三甲酸三乙酯)。以元素硒为催化剂,可以很好地耐受各种芳基/烷基胺,从而在温和的反应条件下以中等至极好的收率得到相应的3,4-二氢喹唑啉-2(1H)-一产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号