当前位置:
X-MOL 学术
›
Nat. Biomed. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy.
Nature Biomedical Engineering ( IF 28.1 ) Pub Date : 2019-07-22 , DOI: 10.1038/s41551-019-0423-2 Di-Wei Zheng 1 , Xue Dong 1 , Pei Pan 1 , Ke-Wei Chen 1 , Jin-Xuan Fan 1 , Si-Xue Cheng 1 , Xian-Zheng Zhang 1
Nature Biomedical Engineering ( IF 28.1 ) Pub Date : 2019-07-22 , DOI: 10.1038/s41551-019-0423-2 Di-Wei Zheng 1 , Xue Dong 1 , Pei Pan 1 , Ke-Wei Chen 1 , Jin-Xuan Fan 1 , Si-Xue Cheng 1 , Xian-Zheng Zhang 1
Affiliation
|
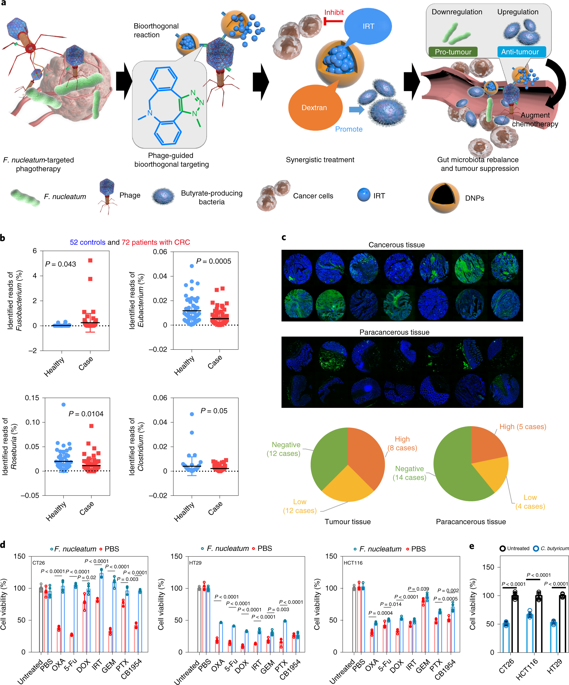
The microbiota in the human gut is strongly correlated with the progression of colorectal cancer (CRC) and with therapeutic responses to CRC. Here, by leveraging the higher concentration of the pro-tumoural Fusobacterium nucleatum and the absence of antineoplastic butyrate-producing bacteria in the faecal microbiota of patients with CRC, we show that-in mice with orthotopic colorectal tumours or with spontaneously formed colorectal tumours-oral or intravenous administration of irinotecan-loaded dextran nanoparticles covalently linked to azide-modified phages that inhibit the growth of F. nucleatum significantly augments the efficiency of first-line chemotherapy treatments of CRC. We also show that oral administration of the phage-guided irinotecan-loaded nanoparticles in piglets led to negligible changes in haemocyte counts, immunoglobulin and histamine levels, and liver and renal functions. Phage-guided nanotechnology for the modulation of the gut microbiota might inspire new approaches for the treatment of CRC.
中文翻译:

结肠直肠癌小鼠模型的肠道菌群的噬菌体指导的调节增强了它们对化学疗法的反应。
人肠道中的微生物群与结直肠癌(CRC)的进展以及对CRC的治疗反应密切相关。在这里,通过利用高浓度的促肿瘤性核梭菌和CRC患者粪便菌群中不存在抗肿瘤的生成丁酸的细菌,我们表明,在患有原位结直肠肿瘤或自发形成结直肠肿瘤的小鼠中,口腔或静脉内施用伊立替康负载的右旋糖酐纳米粒子与叠氮化物修饰的噬菌体共价连接,可抑制F. nucleatum的生长,可显着提高CRC一线化疗的效率。我们还显示,仔猪中噬菌体引导的依立替康负载纳米颗粒的口服给药导致血细胞计数的变化可忽略不计,免疫球蛋白和组胺水平,以及肝和肾功能。噬菌体引导的纳米技术对肠道菌群的调节可能会激发新的治疗CRC的方法。
更新日期:2019-11-18
中文翻译:

结肠直肠癌小鼠模型的肠道菌群的噬菌体指导的调节增强了它们对化学疗法的反应。
人肠道中的微生物群与结直肠癌(CRC)的进展以及对CRC的治疗反应密切相关。在这里,通过利用高浓度的促肿瘤性核梭菌和CRC患者粪便菌群中不存在抗肿瘤的生成丁酸的细菌,我们表明,在患有原位结直肠肿瘤或自发形成结直肠肿瘤的小鼠中,口腔或静脉内施用伊立替康负载的右旋糖酐纳米粒子与叠氮化物修饰的噬菌体共价连接,可抑制F. nucleatum的生长,可显着提高CRC一线化疗的效率。我们还显示,仔猪中噬菌体引导的依立替康负载纳米颗粒的口服给药导致血细胞计数的变化可忽略不计,免疫球蛋白和组胺水平,以及肝和肾功能。噬菌体引导的纳米技术对肠道菌群的调节可能会激发新的治疗CRC的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号